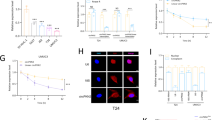
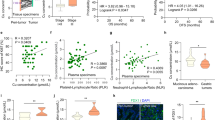
Abstract
Cuproptosis, a cell death process caused by copper ions, is mediated by protein lipidation related to lipoic acid metabolism. There is a close connection between cuproptosis and the progression and prognosis of various tumors. Here, we identified lipoyltransferase 1 (LIPT1), a key gene related to cuproptosis, was downregulated in bladder cancer (BLCA) and was associated with unfavorable patient prognosis. Restoring the LIPT1 expression in BLCA cells suppressed the proliferation and promoted cuproptosis. Moreover, the consequences of RNA sequencing and Bodipy staining showed that the metabolic pathway mediated by LIPT1 inhibited the accumulation of lipid droplets in cells, disrupted endoplasmic reticulum (ER) homeostasis, and promoted cell apoptosis. Additionally, overexpression of LIPT1 not only repressed the proliferation rate of BLCA cells in vitro but also in vivo. Mechanistically, YTH N6-Methyladenosine RNA Binding Protein F2 (YTHDF2) promoted the degradation of LIPT1 mRNA in a m6A-dependent manner. In summary, these conclusions reveal that LIPT1 promotes cuprotosis and ER stress to inhibit the progression of BLCA, indicating that LIPT1 will provide a powerful treatment direction and drug target for treating BLCA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA-sequencing are available at the NCBl Sequence Read Archive (SRA) under the accession number PRJNA1130104.
References
Richters A, Aben KKH, Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895–904. https://doi.org/10.1007/s00345-019-02984-4.
Compérat E, Amin MB, Cathomas R, Choudhury A, De Santis M, Kamat A, et al. Current best practice for bladder cancer: a narrative review of diagnostics and treatments. Lancet (Lond, Engl). 2022;400:1712–21. https://doi.org/10.1016/s0140-6736(22)01188-6.
Grayson M. Bladder cancer. Nature. 2017;551:S33. https://doi.org/10.1038/551S33a.
Chen L, Min J, Wang F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. 2022;7:378. https://doi.org/10.1038/s41392-022-01229-y.
Fukai T, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol. 2018;315:C186–c201. https://doi.org/10.1152/ajpcell.00132.2018.
Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat Commun. 2023;14:6523. https://doi.org/10.1038/s41467-023-42025-8.
Kardos J, Héja L, Simon Á, Jablonkai I, Kovács R, Jemnitz K. Copper signalling: causes and consequences. Cell Commun Signal: CCS. 2018;16:71. https://doi.org/10.1186/s12964-018-0277-3.
Xiao Y, Wang T, Song X, Yang D, Chu Q, Kang YJ. Copper promotion of myocardial regeneration. Exp Biol Med (Maywood, NJ). 2020;245:911–21. https://doi.org/10.1177/1535370220911604.
Sensi SL, Granzotto A, Siotto M, Squitti R. Copper and Zinc Dysregulation in Alzheimer’s Disease. Trends Pharmacol Sci. 2018;39:1049–63. https://doi.org/10.1016/j.tips.2018.10.001.
Rihel J. Copper on the brain. Nat Chem Biol. 2018;14:638–9. https://doi.org/10.1038/s41589-018-0089-1.
Yang H, Liu CN, Wolf RM, Ralle M, Dev S, Pierson H, et al. Obesity is associated with copper elevation in serum and tissues. Metallomics: Integr Biometal Sci. 2019;11:1363–71. https://doi.org/10.1039/c9mt00148d.
Møller LB, Mogensen M, Horn N. Molecular diagnosis of Menkes disease: genotype-phenotype correlation. Biochimie. 2009;91:1273–7. https://doi.org/10.1016/j.biochi.2009.05.011.
Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, et al. Wilson disease. Nat Rev Dis Prim. 2018;4:21. https://doi.org/10.1038/s41572-018-0018-3.
Noda Y, Asada M, Kubota M, Maesako M, Watanabe K, Uemura M, et al. Copper enhances APP dimerization and promotes Aβ production. Neurosci Lett. 2013;547:10–5. https://doi.org/10.1016/j.neulet.2013.04.057.
Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15:174. https://doi.org/10.1186/s13045-022-01392-3.
Denoyer D, Masaldan S, La Fontaine S, Cater MA. Targeting copper in cancer therapy: ‘Copper That Cancer. Metallomics: Integr Biometal Sci. 2015;7:1459–76. https://doi.org/10.1039/c5mt00149h.
Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science (N.Y., NY). 2022;375:1254–61. https://doi.org/10.1126/science.abf0529.
Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol cancer. 2023;22:46. https://doi.org/10.1186/s12943-023-01732-y.
Xu J, Hu Z, Cao H, Zhang H, Luo P, Zhang J, et al. Multi-omics pan-cancer study of cuproptosis core gene FDX1 and its role in kidney renal clear cell carcinoma. Front Immunol. 2022;13:981764. https://doi.org/10.3389/fimmu.2022.981764.
Wen H, Qu C, Wang Z, Gao H, Liu W, Wang H, et al. Cuproptosis enhances docetaxel chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway in prostate cancer. FASEB J: Off Publ Federation Am Societies Exp Biol. 2023;37:e23145. https://doi.org/10.1096/fj.202300980R.
Yang W, Wang Y, Huang Y, Yu J, Wang T, Li C, et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to promote cuproptosis in colorectal cancer. Biomed Pharmacother = Biomedecine pharmacotherapie. 2023;159:114301. https://doi.org/10.1016/j.biopha.2023.114301.
Song W, Yang K, Luo J, Gao Z, Gao Y. Dysregulation of USP18/FTO/PYCR1 signaling network promotes bladder cancer development and progression. Aging. 2021;13:3909–25. https://doi.org/10.18632/aging.202359.
Li J, Xie H, Ying Y, Chen H, Yan H, He L, et al. YTHDF2 mediates the mRNA degradation of the tumor suppressors to induce AKT phosphorylation in N6-methyladenosine-dependent way in prostate cancer. Mol cancer. 2020;19:152. https://doi.org/10.1186/s12943-020-01267-6.
Gao Q, Zheng J, Ni Z, Sun P, Yang C, Cheng M, et al. The m(6)A Methylation-Regulated AFF4 Promotes Self-Renewal of Bladder Cancer Stem Cells. Stem cells Int. 2020;2020:8849218. https://doi.org/10.1155/2020/8849218.
An Y, Duan H. The role of m6A RNA methylation in cancer metabolism. Mol cancer. 2022;21:14. https://doi.org/10.1186/s12943-022-01500-4.
Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochimica et biophysica acta. 2013;1833:3460–70. https://doi.org/10.1016/j.bbamcr.2013.06.028.
Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, et al. HIF2α-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 2015;5:652–67. https://doi.org/10.1158/2159-8290.Cd-14-1507.
Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin M, Manning BD, et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol cell. 2008;29:541–51. https://doi.org/10.1016/j.molcel.2007.12.023.
Liu WQ, Lin WR, Yan L, Xu WH, Yang J. Copper homeostasis and cuproptosis in cancer immunity and therapy. Immunol Rev. 2024;321:211–27. https://doi.org/10.1111/imr.13276.
Liu H, Tang T. Pan-cancer genetic analysis of cuproptosis and copper metabolism-related gene set. Front Oncol. 2022;12:952290. https://doi.org/10.3389/fonc.2022.952290.
Song Q, Zhou R, Shu F, Fu W. Cuproptosis scoring system to predict the clinical outcome and immune response in bladder cancer. Front Immunol. 2022;13:958368. https://doi.org/10.3389/fimmu.2022.958368.
Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat Rev Cancer. 2020;20:303–22. https://doi.org/10.1038/s41568-020-0253-2.
Deng X, Su R, Weng H, Huang H, Li Z, Chen J. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–17. https://doi.org/10.1038/s41422-018-0034-6.
Wang T, Kong S, Tao M, Ju S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol Cancer. 2020;19:88. https://doi.org/10.1186/s12943-020-01204-7.
Liu Q. Current Advances in N6-Methyladenosine Methylation Modification During Bladder Cancer. Front Genet. 2021;12:825109. https://doi.org/10.3389/fgene.2021.825109.
Liu L, Li H, Hu D, Wang Y, Shao W, Zhong J, et al. Insights into N6-methyladenosine and programmed cell death in cancer. Mol cancer. 2022;21:32. https://doi.org/10.1186/s12943-022-01508-w.
Chen X, Zhou X, Wang X. m(6)A binding protein YTHDF2 in cancer. Exp Hematol Oncol. 2022;11:21. https://doi.org/10.1186/s40164-022-00269-y.
Zhang L, Li Y, Zhou L, Zhou H, Ye L, Ou T, et al. The m6A Reader YTHDF2 Promotes Bladder Cancer Progression by Suppressing RIG-I-Mediated Immune Response. Cancer Res. 2023;83:1834–50. https://doi.org/10.1158/0008-5472.Can-22-2485.
Zhong Y, Zeng W, Chen Y, Zhu X. The effect of lipid metabolism on cuproptosis-inducing cancer therapy. Biomed Pharmacother = Biomedecine pharmacotherapie. 2024;172:116247. https://doi.org/10.1016/j.biopha.2024.116247.
Chen T, Liang L, Wang Y, Li X, Yang C. Ferroptosis and cuproptposis in kidney Diseases: dysfunction of cell metabolism. Apoptosis: Int J Program cell death. 2024;29:289–302. https://doi.org/10.1007/s10495-023-01928-z.
Zhang W, Wang M, Liu B, Chen H, Tan J, Meng Q, et al. Glutathione Induced In situ Synthesis of Cu Single-Atom Nanozymes with Anaerobic Glycolysis Metabolism Interference for Boosting Cuproptosis. Angewandte Chemie (International ed in English) 2024:e202402397. https://doi.org/10.1002/anie.202402397.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. https://doi.org/10.1158/2159-8290.Cd-21-1059.
Zhou L, Du K, Dai Y, Zeng Y, Luo Y, Ren M, et al. Metabolic reprogramming based on RNA sequencing of gemcitabine-resistant cells reveals the FASN gene as a therapeutic for bladder cancer. J Transl Med. 2024;22:55. https://doi.org/10.1186/s12967-024-04867-8.
Ni M, Solmonson A, Pan C, Yang C, Li D, Notzon A, et al. Functional Assessment of Lipoyltransferase-1 Deficiency in Cells, Mice, and Humans. Cell Rep. 2019;27:1376–86.e6. https://doi.org/10.1016/j.celrep.2019.04.005.
Kastaniotis AJ, Autio KJ, Kerätär JM, Monteuuis G, Mäkelä AM, Nair RR, et al. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochimica et biophysica acta Mol cell Biol lipids. 2017;1862:39–48. https://doi.org/10.1016/j.bbalip.2016.08.011.
Zhou L, Luo Y, Liu Y, Zeng Y, Tong J, Li M, et al. Fatty Acid Oxidation Mediated by Malonyl-CoA Decarboxylase Represses Renal Cell Carcinoma Progression. Cancer Res. 2023;83:3920–39. https://doi.org/10.1158/0008-5472.Can-23-0969.
Wang W, Lu K, Jiang X, Wei Q, Zhu L, Wang X, et al. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J Exp Clin Cancer Res: CR. 2023;42:142. https://doi.org/10.1186/s13046-023-02720-2.
Zhou L, Song Z, Hu J, Liu L, Hou Y, Zhang X, et al. ACSS3 represses prostate cancer progression through downregulating lipid droplet-associated protein PLIN3. Theranostics. 2021;11:841–60. https://doi.org/10.7150/thno.49384.
Park HJ, Son HJ, Sul OJ, Suh JH, Choi HS. 4-Phenylbutyric acid protects against lipopolysaccharide-induced bone loss by modulating autophagy in osteoclasts. Biochem Pharmacol. 2018;151:9–17. https://doi.org/10.1016/j.bcp.2018.02.019.
Acknowledgements
This work was supported by grants from the National Natural Sciences Foundation of China (NO. 82173294 to C.H.G, NO. 82203099 to L.J.Z.), the Training Program for Middle-aged and Young Discipline Leaders of Health of Henan Province (NO. HNSWJW-2021004 to C.H.G.); the Key Program Jointly Built by Henan Province and the Ministry of Medical Science and Technology(NO.SBGJ202102127 to C.H.G. and SBGJ202102095 to F.Y.T.); the Training Program of Young and Middle-aged Health Science and Technology Innovation Excellent Youth (NO.YXKC2021033 to C.H.G.); the Program of International Training of High-level Talents of Henan Province (NO.202207 to C.H.G.); the Science and Technology Research and Development Plan Joint Foundation of Henan Province (NO. 222301420017 to C.H.G.); the Key Project of Research and Practice of Education and Teaching Reform of Zhengzhou University (NO. 2022ZZUJG082 to C.H.G.); the Professional Degree Graduate Quality Teaching Case Project of Henan Province (NO. YJS2023AL013 to C.H.G.); the Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (NO. QNCXTD2023023 to C.H.G.); the Key Technologies R & D Program of Henan Province (NO. 232102521032 to C.H.G.); the Basic Research Incubation Program for Young Teachers of Zhengzhou University (NO. JC21854035 to F.Y.T.); the Joint Construction Project between Medical Science and Technology Research Project of Henan Province (No. LHGJ20220335 to L.J.Z.); the Key Scientific Research projects of Colleges and Universities of Henan Province (NO. 24A320058 to F.Y.T.).
Author information
Authors and Affiliations
Contributions
C.H.G., F.Y.T., and L.J.Z. conceived the study. K.X.D., Y.B.L. and L.Z. designed experiments. K.X.D., Y.B.L., and Y.M.Z. performed experiments. K.X.D., Y.B.L. and Y.H.D. assisted with animal experiments. M.D.R., Y.H.L., and W.B.P. helped to obtain BLCA patients’ clinical information. K.X.D., and Y.B.L. analyzed the data. K.X.D. wrote the manuscript and all authors reviewed and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, K., Luo, Y., Zhang, L. et al. m6A modification of lipoyltransferase 1 inhibits bladder cancer progression by activating cuproptosis. Oncogene (2024). https://doi.org/10.1038/s41388-024-03139-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-024-03139-5