Abstract
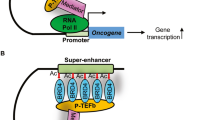
NUT carcinoma (NC) is an extremely aggressive squamous cancer with no effective therapy. NC is driven, most commonly, by the BRD4-NUT fusion oncoprotein. BRD4-NUT combines the chromatin-binding bromo- and extraterminal domain-containing (BET) protein, BRD4, with an unstructured, poorly understood protein, NUT, which recruits and activates the histone acetyltransferase p300. Recruitment of p300 to chromatin by BRD4 is believed to lead to the formation of hyperacetylated nuclear foci, as seen by immunofluorescence. BRD4-NUT nuclear foci correspond with massive contiguous regions of chromatin co-enriched with BRD4-NUT, p300, and acetylated histones, termed “megadomains” (MD). Megadomains stretch for as long as 2 MB. Proteomics has defined a BRD4-NUT chromatin complex in which members that associate with BRD4 also exist as rare NUT-fusion partners. This suggests that the common pathogenic denominator is the presence of both BRD4 and NUT, and that the function of BRD4-NUT may mimic that of wild-type BRD4. If so, then MDs may function as massive super-enhancers, activating transcription in a BET-dependent manner. Common targets of MDs across multiple NCs and tissues are three stem cell-related transcription factors frequently implicated in cancer: MYC, SOX2, and TP63. Recently, MDs were found to form a novel nuclear sub-compartment, called subcompartment M (subM), where MD-MD interactions occur both intra- and inter-chromosomally. Included in subM are MYC, SOX2, and TP63. Here we explore the possibility that if MDs are simply large super-enhancers, subM may exist in other cell systems, with broad implications for how 3D organization of the genome may function in gene regulation and maintenance of cell identity. Finally, we discuss how our knowledge of BRD4-NUT function has been leveraged for the therapeutic development of first-in-class BET inhibitors and other targeted strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline carcinoma of children and young adults with nut rearrangement. J Clin Oncol. 2004;22:4135–9. Epub 2004/10/16.
French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. Brd4-nut fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–7.
Dey A, Ellenberg J, Farina A, Coleman AE, Maruyama T, Sciortino S, et al. A bromodomain protein, mcap, associates with mitotic chromosomes and affects g(2)-to-m transition. Mol Cell Biol. 2000;20:6537–49.
Shiota H, Barral S, Buchou T, Tan M, Coute Y, Charbonnier G, et al. Nut directs p300-dependent, genome-wide h4 hyperacetylation in male germ cells. Cell Rep. 2018;24:3477–87. e6.
Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci USA. 2003;100:8758–63.
Reynoird N, Schwartz BE, Delvecchio M, Sadoul K, Meyers D, Mukherjee C. et al. Oncogenesis by sequestration of cbp/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–52. Epub 2010/08/03.
French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. Brd-nut oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–42. Epub 2007/10/16.
Alekseyenko AA, Walsh EM, Zee BM, Pakozdi T, Hsi P, Lemieux ME, et al. Ectopic protein interactions within brd4-chromatin complexes drive oncogenic megadomain formation in nut midline carcinoma. Proc Natl Acad Sci USA. 2017;114:E4184–92. Epub 2017/05/10.
Shiota H, Elya JE, Alekseyenko A, Chou PM, Gorman SA, Barbash O. et al. Z4’ complex member fusions in nut carcinoma: Implications for a novel oncogenic mechanism. Mol Cancer Res. 2018;16:1826–33.
French CA, Rahman S, Walsh EM, Kuhnle S, Grayson AR, Lemieux ME, et al. Nsd3-nut fusion oncoprotein in nut midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014;4:928–41. Epub 2014/05/31.
Chau NG, Ma C, Danga K, Al-Sayegh H, Nardi V, Barrette R. et al. An anatomical site and genetic based prognostic model for patients with nut midline carcinoma: analysis of 124 patients. JNCI Cancer Spectrum. 2019;4:pkz094.
Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Luer SC, et al. Clinicopathologic features and long-term outcomes of nut midline carcinoma. Clin Cancer Res. 2012;18:5773–9. Epub 2012/08/17.
Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000–2019. JAMA Oncol. 2020. Epub 2020/08/08.
Wang R, Liu W, Helfer CM, Bradner JE, Hornick JL, Janicki SM, et al. Activation of sox2 expression by brd4-nut oncogenic fusion drives neoplastic transformation in nut midline carcinoma. Cancer Res. 2014;74:3332–43 .
Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. Myc, a downstream target of brd-nut, is necessary and sufficient for the blockade of differentiation in nut midline carcinoma. Oncogene. 2014;33:1736–42. Epub 2013/04/23.
Stirnweiss A, Oommen J, Kotecha RS, Kees UR, Beesley AH. Molecular-genetic profiling and high-throughput in vitro drug screening in nut midline carcinoma-an aggressive and fatal disease. Oncotarget. 2017;8:112313–29 .
Lee JK, Louzada S, An Y, Kim SY, Kim S, Youk J. et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in nut midline carcinoma. Ann Oncol. 2017;28:890–7. PMC5378225.
Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T. et al. Clinical response of carcinomas harboring the brd4-nut oncoprotein to the targeted bromodomain inhibitor otx015/mk-8628. Cancer Discov. 2016;6:492–500. Epub 2016/03/16.
Alekseyenko AA, Walsh EM, Wang X, Grayson AR, Hsi PT, Kharchenko PV, et al. The oncogenic brd4-nut chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29:1507–23. Epub 2015/07/30.
Wang R, You J. Mechanistic analysis of the role of bromodomain-containing protein 4 (brd4) in brd4-nut oncoprotein-induced transcriptional activation. J Biol Chem. 2015;290:2744–58.
Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, et al. From silencing to gene expression: real-time analysis in single cells. Cell. 2004;116:683–98. Epub 2004/03/10.
Alekseyenko AA, McElroy KA, Kang H, Zee BM, Kharchenko PV, Kuroda MI. Biotap-xl: cross-linking/tandem affinity purification to study DNA targets, rna, and protein components of chromatin-associated complexes. Curr Protoc Mol Biol. 2015;109:21 30 1–21 30 2. Epub 2015/01/07.
Fong CY, Gilan O, Lam EY, Rubin AF, Ftouni S, Tyler D, et al. Bet inhibitor resistance emerges from leukaemia stem cells. Nature. 2015;525:538–42. Epub 2015/09/15.
Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, et al. The brd4 extraterminal domain confers transcription activation independent of ptefb by recruiting multiple proteins, including nsd3. Mol Cell Biol. 2011;31:2641–52. Epub 2011/05/11.
Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. Bet bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol Cell. 2015;58:1028–39. PMC4475489.
Malovannaya A, Lanz RB, Jung SY, Bulynko Y, Le NT, Chan DW, et al. Analysis of the human endogenous coregulator complexome. Cell. 2011;145:787–99.
Spruijt CG, Luijsterburg MS, Menafra R, Lindeboom RG, Jansen PW, Edupuganti RR, et al. Zmynd8 co-localizes with nurd on target genes and regulates poly(adp-ribose)-dependent recruitment of gatad2a/nurd to sites of DNA damage. Cell Rep. 2016;17:783–98.
Li N, Li Y, Lv J, Zheng X, Wen H, Shen H, et al. Zmynd8 reads the dual histone mark h3k4me1-h3k14ac to antagonize the expression of metastasis-linked genes. Mol Cell. 2016;63:470–84 .
Yang Z, Yik JH, Chen R, He N, Jang MK, Ozato K, et al. Recruitment of p-tefb for stimulation of transcriptional elongation by the bromodomain protein brd4. Mol Cell. 2005;19:535–45.
Yang Z, He N, Zhou Q. Brd4 recruits p-tefb to chromosomes at late mitosis to promote g1 gene expression and cell cycle progression. Mol Cell Biol. 2008;28:967–76.
Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Lawrence JB, et al. Molecular cloning and functional analysis of the adenovirus e1a-associated 300-kd protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–84. Epub 1994/04/15.
Ortega E, Rengachari S, Ibrahim Z, Hoghoughi N, Gaucher J, Holehouse AS, et al. Transcription factor dimerization activates the p300 acetyltransferase. Nature. 2018;562:538–44. Epub 2018/10/17.
Talbot D, Collis P, Antoniou M, Vidal M, Grosveld F, Greaves DR. A dominant control region from the human beta-globin locus conferring integration site-independent gene expression. Nature. 1989;338:352–5. Epub 1989/03/23.
Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34. Epub 2013/04/16.
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–19. Epub 2013/04/16.
Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–47. Epub 2013/10/15.
Jang MK, Mochizuki K, Zhou M, Jeong HS, Brady JN, Ozato K. The bromodomain protein brd4 is a positive regulatory component of p-tefb and stimulates rna polymerase ii-dependent transcription. Mol Cell. 2005;19:523–34.
Di Micco R, Fontanals-Cirera B, Low V, Ntziachristos P, Yuen SK, Lovell CD, et al. Control of embryonic stem cell identity by brd4-dependent transcriptional elongation of super-enhancer-associated pluripotency genes. Cell Rep. 2014;9:234–47. Epub 2014/09/30.
Wang D, Garcia-Bassets I, Benner C, Li W, Su X, Zhou Y, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by erna. Nature. 2011;474:390–4. Epub 2011/05/17.
McCleland ML, Mesh K, Lorenzana E, Chopra VS, Segal E, Watanabe C, et al. Ccat1 is an enhancer-templated rna that predicts bet sensitivity in colorectal cancer. J Clin Invest. 2016;126:639–52. Epub 2016/01/12.
Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, et al. Human colorectal cancer-specific ccat1-l lncrna regulates long-range chromatin interactions at the myc locus. Cell Res. 2014;24:513–31. Epub 2014/03/26.
Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, et al. Pvt1 dependence in cancer with myc copy-number increase. Nature. 2014;512:82–6. Epub 2014/07/22.
Chapuy B, McKeown MR, Lin CY, Monti S, Roemer MG, Qi J, et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large b cell lymphoma. Cancer Cell. 2013;24:777–90. Epub 2013/12/18.
Wang H, Zang C, Taing L, Arnett KL, Wong YJ, Pear WS, et al. Notch1-rbpj complexes drive target gene expression through dynamic interactions with superenhancers. Proc Natl Acad Sci USA. 2014;111:705–10. Epub 2014/01/01.
Herranz D, Ambesi-Impiombato A, Palomero T, Schnell SA, Belver L, Wendorff AA, et al. A notch1-driven myc enhancer promotes t cell development, transformation and acute lymphoblastic leukemia. Nat Med. 2014;20:1130–7. Epub 2014/09/10.
Zhang X, Zegar T, Lucas A, Morrison-Smith C, Knox T, French CA, et al. Therapeutic targeting of p300/cbp hat domain for the treatment of nut midline carcinoma. Oncogene. 2020;39:4770–9. Epub 2020/05/06.
Guo L, Li J, Zeng H, Guzman AG, Li T, Lee M, et al. A combination strategy targeting enhancer plasticity exerts synergistic lethality against beti-resistant leukemia cells. Nat Commun. 2020;11:740. Epub 2020/02/08.
Vito D, Eriksen JC, Skjodt C, Weilguny D, Rasmussen SK, Smales CM. Defining IncRNAs correlated with CHO cell growth and IgG productivity by RNA-SEQ. iScience. 2020;23:100785. Epub 2020/01/22.
Riquelme E, Suraokar MB, Rodriguez J, Mino B, Lin HY, Rice DC, et al. Frequent coamplification and cooperation between c-myc and pvt1 oncogenes promote malignant pleural mesothelioma. J Thorac Oncol. 2014;9:998–1007. Epub 2014/06/14.
Bouchard C, Thieke K, Maier A, Saffrich R, Hanley-Hyde J, Ansorge W, et al. Direct induction of cyclin d2 by myc contributes to cell cycle progression and sequestration of p27. EMBO J. 1999;18:5321–33. Epub 1999/10/03.
Warner BJ, Blain SW, Seoane J, Massague J. MYC downregulation by transforming growth factor beta required for activation of the p15(ink4b) g(1) arrest pathway. Mol Cell Biol. 1999;19:5913–22. Epub 1999/08/24.
Mateyak MK, Obaya AJ, Sedivy JM. C-myc regulates cyclin d-CDK4 and -CDK6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19:4672–83. Epub 1999/06/22.
Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA. 2000;97:2229–34. Epub 2000/02/26.
Miliani de Marval PL, Macias E, Rounbehler R, Sicinski P, Kiyokawa H, Johnson DG, et al. Lack of cyclin-dependent kinase 4 inhibits c-myc tumorigenic activities in epithelial tissues. Mol Cell Biol. 2004;24:7538–47. Epub 2004/08/18.
Daksis JI, Lu RY, Facchini LM, Marhin WW, Penn LJ. Myc induces cyclin d1 expression in the absence of de novo protein synthesis and links mitogen-stimulated signal transduction to the cell cycle. Oncogene. 1994;9:3635–45. Epub 1994/12/01.
Steiner P, Philipp A, Lukas J, Godden-Kent D, Pagano M, Mittnacht S, et al. Identification of a myc-dependent step during the formation of active g1 cyclin-cdk complexes. EMBO J. 1995;14:4814–26. Epub 1995/10/02.
Liao S, Maertens O, Cichowski K, Elledge SJ. Genetic modifiers of the brd4-nut dependency of nut midline carcinoma uncovers a synergism between betis and cdk4/6is. Genes Dev. 2018;32:1188–200.
Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. P63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–16. Epub 1998/10/17.
Giacobbe A, Compagnone M, Bongiorno-Borbone L, Antonov A, Markert EK, Zhou JH, et al. P63 controls cell migration and invasion by transcriptional regulation of mtss1. Oncogene. 2016;35:1602–8. Epub 2015/06/30.
Latina A, Viticchie G, Lena AM, Piro MC, Annicchiarico-Petruzzelli M, Melino G, et al. Deltanp63 targets cytoglobin to inhibit oxidative stress-induced apoptosis in keratinocytes and lung cancer. Oncogene. 2016;35:1493–503. Epub 2015/06/23.
Hamdan FH, Johnsen SA. Deltanp63-dependent super enhancers define molecular identity in pancreatic cancer by an interconnected transcription factor network. Proc Natl Acad Sci USA. 2018;115:E12343–E52. Epub 2018/12/14.
Abbas HA, Bui NHB, Rajapakshe K, Wong J, Gunaratne P, Tsai KY, et al. Distinct tp63 isoform-driven transcriptional signatures predict tumor progression and clinical outcomes. Cancer Res. 2018;78:451–62. Epub 2017/11/29.
Westcott JM, Camacho S, Nasir A, Huysman ME, Rahhal R, Dang TT, et al. Deltanp63-regulated epithelial-to-mesenchymal transition state heterogeneity confers a leader-follower relationship that drives collective invasion. Cancer Res. 2020;80:3933–44. Epub 2020/07/15.
Tilson MP, Bishop JA. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head Neck Pathol. 2014;8:141–5.
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. Sox2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–42. Epub 2009/10/06.
Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, et al. Sox2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE. 2010;5:e8960. Epub 2010/02/04.
Mukhopadhyay A, Berrett KC, Kc U, Clair PM, Pop SM, Carr SR, et al. Sox2 cooperates with lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 2014;8:40–9. Epub 2014/06/24.
Chen Y, Li Y, Peng Y, Zheng X, Fan S, Yi Y, et al. Deltanp63alpha down-regulates c-myc modulator mm1 via e3 ligase herc3 in the regulation of cell senescence. Cell Death Differ. 2018;25:2118–29. Epub 2018/06/09.
Alexandrova EM, Petrenko O, Nemajerova A, Romano RA, Sinha S, Moll UM. Deltanp63 regulates select routes of reprogramming via multiple mechanisms. Cell Death Differ. 2013;20:1698–708. Epub 2013/09/10.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. Epub 2006/08/15.
Watanabe H, Ma Q, Peng S, Adelmant G, Swain D, Song W, et al. Sox2 and p63 colocalize at genetic loci in squamous cell carcinomas. J Clin Invest. 2014;124:1636–45. Epub 2014/03/05.
Chiang CT, Chu WK, Chow SE, Chen JK. Overexpression of delta np63 in a human nasopharyngeal carcinoma cell line downregulates ckis and enhances cell proliferation. J Cell Physiol. 2009;219:117–22. Epub 2008/12/18.
Lanza M, Marinari B, Papoutsaki M, Giustizieri ML, D’Alessandra Y, Chimenti S, et al. Cross-talks in the p53 family: Deltanp63 is an anti-apoptotic target for deltanp73alpha and p53 gain-of-function mutants. Cell Cycle. 2006;5:1996–2004. Epub 2006/08/26.
Gillespie MA, Palii CG, Sanchez-Taltavull D, Shannon P, Longabaugh WJR, Downes DJ, et al. Absolute quantification of transcription factors reveals principles of gene regulation in erythropoiesis. Mol Cell. 2020;78:960–74. e11. Epub 2020/04/25.
Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–93. Epub 2009/10/10.
Rao SS, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3d map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–80. Epub 2014/12/17.
Rosencrance CD, Ammouri HN, Yu Q, Ge T, Rendleman EJ, Marshall SA, et al. Chromatin hyperacetylation impacts chromosome folding by forming a nuclear subcompartment. Mol Cell. 2020;78:112–26. e12. Epub 2020/04/04.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56. Epub 2005/09/13.
Saint-Andre V, Federation AJ, Lin CY, Abraham BJ, Reddy J, Lee TI, et al. Models of human core transcriptional regulatory circuitries. Genome Res. 2016;26:385–96. Epub 2016/02/05.
Wu T, Kamikawa YF, Donohoe ME. Brd4’s bromodomains mediate histone h3 acetylation and chromatin remodeling in pluripotent cells through p300 and brg1. Cell Rep. 2018;25:1756–71.
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of bet bromodomains. Nature. 2010;468:1067–73. Epub 2010/09/28.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. Bet bromodomain inhibition as a therapeutic strategy to target c-myc. Cell. 2011;146:904–17. Epub 2011/09/06.
Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting myc dependence in cancer by inhibiting bet bromodomains. Proc Natl Acad Sci USA. 2011;108:16669–74. Epub 2011/09/29.
Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of bet recruitment to chromatin as an effective treatment for mll-fusion leukaemia. Nature. 2011;478:529–33. Epub 2011/10/04.
Wyce A, Ganji G, Smitheman KN, Chung CW, Korenchuk S, Bai Y, et al. Bet inhibition silences expression of mycn and bcl2 and induces cytotoxicity in neuroblastoma tumor models. PLoS ONE. 2013;8:e72967. Epub 2013/09/07.
Henssen A, Thor T, Odersky A, Heukamp L, El-Hindy N, Beckers A, et al. Bet bromodomain protein inhibition is a therapeutic option for medulloblastoma. Oncotarget. 2013;4:2080–95. Epub 2013/11/16.
Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH, et al. Targeting mycn in neuroblastoma by bet bromodomain inhibition. Cancer Discov. 2013;3:308–23 .
Qiu H, Jackson AL, Kilgore JE, Zhong Y, Chan LL, Gehrig PA, et al. Jq1 suppresses tumor growth through downregulating ldha in ovarian cancer. Oncotarget. 2015;6:6915–30. Epub 2015/03/13.
Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of bet bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–82. Epub 2014/04/25.
Lewin J, Soria JC, Stathis A, Delord JP, Peters S, Awada A. et al. Phase ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol. 2018;36:3007–3014.
Piha-Paul SA, Hann CL, French CA, Cousin S, Bra¤a I, Cassier PA. et al. Phase 1 study of molibresib (gsk525762), a bromodomain and extra-terminal domain protein inhibitor, in nut carcinoma and other solid tumors. JNCI Cancer Spectrum. 2019;4:pkz093.
Coude MM, Braun T, Berrou J, Dupont M, Bertrand S, Masse A, et al. Bet inhibitor otx015 targets brd2 and brd4 and decreases c-myc in acute leukemia cells. Oncotarget. 2015. Epub 2015/05/21.
Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE, et al. Small-molecule inhibition of brdt for male contraception. Cell. 2012;150:673–84. Epub 2012/08/21.
Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–23. Epub 2010/11/12.
Sheppard GS, Wang L, Fidanze SD, Hasvold LA, Liu D, Pratt JK, et al. Discovery of n-ethyl-4-[2-(4-fluoro-2,6-dimethyl-phenoxy)-5-(1-hydroxy-1-methyl-ethyl)phenyl]-6-methyl-7-oxo-1h-pyrrolo[2,3-c]pyridine-2-carboxamide (abbv-744), a bet bromodomain inhibitor with selectivity for the second bromodomain. J Med Chem. 2020;63:5585–623. Epub 2020/04/24.
Faivre EJ, McDaniel KF, Albert DH, Mantena SR, Plotnik JP, Wilcox D, et al. Selective inhibition of the bd2 bromodomain of bet proteins in prostate cancer. Nature. 2020;578:306–10. Epub 2020/01/24.
Morrison-Smith CD, Knox TM, Filic I, Soroko KM, Eschle BK, Wilkens MK, et al. Combined targeting of the brd4-nut-p300 axis in nut midline carcinoma by dual selective bromodomain inhibitor, neo2734. Mol Cancer Ther. 2020;19:1406–14. Epub 2020/05/07.
Lasko LM, Jakob CG, Edalji RP, Qiu W, Montgomery D, Digiammarino EL, et al. Discovery of a selective catalytic p300/cbp inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–32.
Romero FA, Murray J, Lai KW, Tsui V, Albrecht BK, An L, et al. Gne-781, a highly advanced potent and selective bromodomain inhibitor of cyclic adenosine monophosphate response element binding protein, binding protein (cbp). J Med Chem. 2017;60:9162–83.
Yan Y, Ma J, Wang D, Lin D, Pang X, Wang S, et al. The novel bet-cbp/p300 dual inhibitor neo2734 is active in spop mutant and wild-type prostate cancer. EMBO Mol Med. 2019;11:e10659. Epub 2019/09/29.
Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, et al. Differentiation of nut midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–96. Epub 2011/03/31.
Maher OM, Christensen AM, Yedururi S, Bell D, Tarek N. Histone deacetylase inhibitor for nut midline carcinoma. Pediatr Blood Cancer. 2015;62:715–7.
Bragelmann J, Dammert MA, Dietlein F, Heuckmann JM, Choidas A, Bohm S, et al. Systematic kinase inhibitor profiling identifies cdk9 as a synthetic lethal target in nut midline carcinoma. Cell Rep. 2017;20:2833–45. Epub 2017/09/21.
Beesley AH, Stirnweiss A, Ferrari E, Endersby R, Howlett M, Failes TW, et al. Comparative drug screening in nut midline carcinoma. Br J Cancer. 2014;110:1189–98. Epub 2014/02/13.
Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of nut midline carcinoma using a nut-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–91. Epub 2009/04/14.
French CA, Bakker MAD Who classification of head and neck tumours. 4th ed. El-Naggar A, Chan JKC, Grandis JR, Takata T, Slootweg P, editors. Lyon: International Agency for Research on Cancer (IARC); 2017.
French CA. Nut carcinoma: clinicopathologic features, pathogenesis, and treatment. Pathol Int. 2018;68:583–95. Epub 2018/10/27.
Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon KR. et al. Cohesin loss eliminates all loop domains. Cell. 2017;171:305–20. e24.
Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell.2017;171:1678–91. e13.
Hinze L, Pfirrmann M, Karim S, Degar J, McGuckin C, Vinjamur D. et al. Synthetic lethality of wnt pathway activation and asparaginase in drug-resistant acute leukemias. Cancer Cell. 2019;35:664–76.e7. Epub 2019/04/17.
Rizk M, Tuzmen S. Patisiran for the treatment of patients with familial amyloid polyneuropathy. Drugs Today (Barc). 2019;55:315–27. Epub 2019/05/28.
Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, Hsu PD. Transcriptome engineering with rna-targeting type vi-d crispr effectors. Cell. 2018;173:665–76. e14. Epub 2018/03/20.
Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–55. Epub 2020/01/17.
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–5. Epub 2020/01/17.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
CAF receives consultation and research funding from Boehringer-Ingelheim. KPE declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eagen, K.P., French, C.A. Supercharging BRD4 with NUT in carcinoma. Oncogene 40, 1396–1408 (2021). https://doi.org/10.1038/s41388-020-01625-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01625-0
This article is cited by
-
Znf687 recruits Brd4-Smrt complex to regulate gfi1aa during neutrophil development
Leukemia (2024)
-
Molecular features driving condensate formation and gene expression by the BRD4-NUT fusion oncoprotein are overlapping but distinct
Scientific Reports (2023)
-
Structural mechanism of BRD4-NUT and p300 bipartite interaction in propagating aberrant gene transcription in chromatin in NUT carcinoma
Nature Communications (2023)
-
Structural insights into p300 regulation and acetylation-dependent genome organisation
Nature Communications (2022)
-
Thyroid Carcinoma with NSD3::NUTM1 Fusion: a Case with Thyrocyte Differentiation and Colloid Production
Endocrine Pathology (2022)