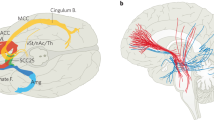
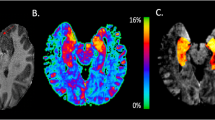
Abstract
Neuromodulation is increasingly becoming a therapeutic option for treatment resistant psychiatric disorders. These non-invasive and invasive therapies are still being refined but are clinically effective and, in some cases, provide sustained symptom reduction. Neuromodulation relies on changing activity within a specific brain region or circuit, but the precise mechanisms of action of these therapies, is unclear. Here we review work in both humans and animals that has provided insight into how therapies such as deep brain and transcranial magnetic stimulation alter neural activity across the brain. We focus on studies that have combined neuromodulation with neuroimaging such as PET and MRI as these measures provide detailed information about the distributed networks that are modulated and thus insight into both the mechanisms of action of neuromodulation but also potentially the basis of psychiatric disorders. Further we highlight work in nonhuman primates that has revealed how neuromodulation changes neural activity at different scales from single neuron activity to functional connectivity, providing key insight into how neuromodulation influences the brain. Ultimately, these studies highlight the value of combining neuromodulation with neuroimaging to reveal the mechanisms through which these treatments influence the brain, knowledge vital for refining targeted neuromodulation therapies for psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Figee M, Riva-Posse P, Choi KS, Bederson L, Mayberg HS, Kopell BH. Deep brain stimulation for depression. Neurotherapeutics. 2022;19:1229–45.
Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci. 2011;34:289–307.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60.
Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry. 2018;84:28–37.
Herrera-Melendez A-L, Bajbouj M, Aust S. Application of transcranial direct current stimulation in psychiatry. Neuropsychobiology. 2020;79:372–83.
Moffa AH, Brunoni AR, Nikolin S, Loo CK. Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am. 2018;41:447–63.
Riis TS, Feldman DA, Vonesh LC, Brown JR, Solzbacher D, Kubanek J, et al. Durable effects of deep brain ultrasonic neuromodulation on major depression: a case report. J Med Case Rep. 2023;17:449.
Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014. https://doi.org/10.1016/j.biopsych.2014.03.029.
Cole EJ, Phillips AL, Bentzley BS, Stimpson KH, Nejad R, Barmak F, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179:132–41.
Baldermann JC, Schüller T, Kohl S, Voon V, Li N, Hollunder B, et al. Connectomic deep brain stimulation for obsessive-compulsive disorder. Biol Psychiatry. 2021;90:678–88.
Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, et al. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am J Psychiatry. 2019;176:949–56.
Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry. 2017;4:839–49.
Kopell B, Himes L, Ross E, Mayberg H. ID: 221667 lessons in BROADEN and since BROADEN: the long and winding path to FDA breakthrough designation. Neuromodulation. 2023;26:S5.
Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex. 2008;18:1374–83.
Riva-Posse P, Choi KS, Holtzheimer PE, Crowell AL, Garlow SJ, Rajendra JK, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2017. https://doi.org/10.1038/mp.2017.59.
Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82.
Sale MV, Mattingley JB, Zalesky A, Cocchi L. Imaging human brain networks to improve the clinical efficacy of non-invasive brain stimulation. Neurosci Biobehav Rev. 2015;57:187–98.
Rudebeck PH, Rich EL, Mayberg HS. From bed to bench side: reverse translation to optimize neuromodulation for mood disorders. Proc Natl Acad Sci USA. 2019;116:2688–96.
Denys D, Mantione M, Figee M, van den Munckhof P, Koerselman F, Westenberg H, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67:1061–8.
Williams NR, Sudheimer KD, Cole EJ, Varias AD, Goldstein-Piekarski AN, Stetz P, et al. Accelerated neuromodulation therapy for obsessive-compulsive disorder. Brain Stimul. 2021;14:435–7.
Gault JM, Davis R, Cascella NG, Saks ER, Corripio-Collado I, Anderson WS, et al. Approaches to neuromodulation for schizophrenia. J Neurol Neurosurg Psychiatry. 2018;89:777–87.
Hensel L, Lüdtke J, Brouzou KO, Eickhoff SB, Kamp D, Schilbach L. Noninvasive brain stimulation in autism: review and outlook for personalized interventions in adult patients. Cereb Cortex. 2024;34:8–18.
Mehta DD, Praecht A, Ward HB, Sanches M, Sorkhou M, Tang VM, et al. A systematic review and meta-analysis of neuromodulation therapies for substance use disorders. Neuropsychopharmacology. 2024;49:649–80.
Jensen MP, Day MA, Miró J. Neuromodulatory treatments for chronic pain: efficacy and mechanisms. Nat Rev Neurol. 2014;10:167–78.
Knotkova H, Hamani C, Sivanesan E, Le Beuffe MFE, Moon JY, Cohen SP, et al. Neuromodulation for chronic pain. Lancet. 2021;397:2111–24.
Denison T, Morrell MJ. Neuromodulation in 2035: the neurology future forecasting series. Neurology. 2022;98:65–72.
Wiseman M, Sewell IJ, Nestor SM, Giacobbe P, Hamani C, Lipsman N, et al. Cognitive effects of focal neuromodulation in neurological and psychiatric disorders. Nat Rev Psychol. 2024;3:242–60.
Friedrich MJ. Depression is the leading cause of disability around the World. JAMA. 2017;317:1517.
Zhdanava M, Pilon D, Ghelerter I, Chow W, Joshi K, Lefebvre P, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82:20m13699.
Thomas L, Kessler D, Campbell J, Morrison J, Peters TJ, Williams C, et al. Prevalence of treatment-resistant depression in primary care: cross-sectional data. Br J Gen Pract. 2013;63:e852–858.
Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28:261–74.
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7.
Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61.
Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43.
Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry. 2005;58:843–53.
Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14.
Dunlop BW, Rajendra JK, Craighead WE, Kelley ME, McGrath CL, Choi KS, et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am J Psychiatry. 2017;174:533–45.
Hadas I, Sun Y, Lioumis P, Zomorrodi R, Jones B, Voineskos D, et al. Association of repetitive transcranial magnetic stimulation treatment with subgenual cingulate hyperactivity in patients with major depressive disorder: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2019;2:e195578.
Sendi MSE, Waters AC, Tiruvadi V, Riva-Posse P, Crowell A, Isbaine F, et al. Intraoperative neural signals predict rapid antidepressant effects of deep brain stimulation. Transl Psychiatry. 2021;11:551.
Alagapan S, Choi KS, Heisig S, Riva-Posse P, Crowell A, Tiruvadi V, et al. Cingulate dynamics track depression recovery with deep brain stimulation. Nature. 2023;622:130–8.
Drobisz D, Damborska A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266–73.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Pascual-Leone A, Rubio B, Pallardó F, Catalá MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–7.
Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–34.
Janicak PG, Nahas Z, Lisanby SH, Solvason HB, Sampson SM, McDonald WM, et al. Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: assessment of relapse during a 6-month, multisite, open-label study. Brain Stimul. 2010;3:187–99.
Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603.
Cash RFH, Zalesky A, Thomson RH, Tian Y, Cocchi L, Fitzgerald PB. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry. 2019;86:e5–7.
Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. 2020;177:716–26.
Vos T, Mathers CD. The burden of mental disorders: a comparison of methods between the Australian burden of disease studies and the Global Burden of Disease study. Bull World Health Organ. 2000;78:427–38.
Nuttin B, Wu H, Mayberg H, Hariz M, Gabriels L, Galert T, et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J Neurol Neurosurg Psychiatry. 2014. https://doi.org/10.1136/jnnp-2013-306580.
Talairach J, Hecaen H, David M. Lobotomie préfrontale limitée par électrocoagulation des fibres thalamo-frontales à leur émergence du bras antérieur de la capsule interne. Rev Neurol. 1949;3:59. 8
Ballantine HT, Cassidy WL, Flanagan NB, Marino R. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26:488–95.
Patel SR, Aronson JP, Sheth SA, Eskandar EN. Lesion procedures in psychiatric neurosurgery. World Neurosurg. 2013;80:S31.e9–16.
Sheth SA, Neal J, Tangherlini F, Mian MK, Gentil A, Cosgrove GR, et al. Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg. 2013;118:491–7.
Froudist-Walsh S, Browning PG, Young JJ, Murphy KL, Mars RB, Fleysher L, et al. Macro-connectomics and microstructure predict dynamic plasticity patterns in the non-human primate brain. Elife. 2018;7:e34354.
McLaughlin NCR, Magnotti JF, Banks GP, Nanda P, Hoexter MQ, Lopes AC, et al. Gamma knife capsulotomy for intractable OCD: neuroimage analysis of lesion size, location, and clinical response. Transl Psychiatry. 2023;13:134.
Yu CP, Tsang CP, Ip YM. Gamma knife radiosurgery versus deep brain stimulation for treatment-refractory depression and obsessive-compulsive disorder: a brief comparative summary. Prog Brain Res. 2022;272:33–40.
Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526.
Karas PJ, Lee S, Jimenez-Shahed J, Goodman WK, Viswanathan A, Sheth SA. Deep brain stimulation for obsessive compulsive disorder: evolution of surgical stimulation target parallels changing model of dysfunctional brain circuits. Front Neurosci. 2018;12:998.
Provenza NR, Reddy S, Allam AK, Rajesh SV, Diab N, Reyes G, et al. Disruption of neural periodicity predicts clinical response after deep brain stimulation for obsessive-compulsive disorder. Nat Med. 2024. https://doi.org/10.1038/s41591-024-03125-0.
Greenberg BD, George MS, Martin JD, Benjamin J, Schlaepfer TE, Altemus M, et al. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry. 1997;154:867–9.
Cocchi L, Zalesky A, Nott Z, Whybird G, Fitzgerald PB, Breakspear M. Transcranial magnetic stimulation in obsessive-compulsive disorder: a focus on network mechanisms and state dependence. Neuroimage Clin. 2018;19:661–74.
Price RB, Gillan CM, Hanlon C, Ferrarelli F, Kim T, Karim HT, et al. Effect of experimental manipulation of the orbitofrontal cortex on short-term markers of compulsive behavior: a theta burst stimulation study. Am J Psychiatry. 2021;178:459–68.
Roth Y, Tendler A, Arikan MK, Vidrine R, Kent D, Muir O, et al. Real-world efficacy of deep TMS for obsessive-compulsive disorder: post-marketing data collected from twenty-two clinical sites. J Psychiatr Res. 2021;137:667–72.
Steuber ER, McGuire JF. A meta-analysis of transcranial magnetic stimulation in obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimag. 2023;8:1145–55.
Hynynen K, Jolesz FA. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med Biol. 1998;24:275–83.
Germann J, Elias GJB, Neudorfer C, Boutet A, Chow CT, Wong EHY, et al. Potential optimization of focused ultrasound capsulotomy for obsessive compulsive disorder. Brain. 2021;144:3529–40.
Grover S, Nguyen JA, Viswanathan V, Reinhart RMG. High-frequency neuromodulation improves obsessive-compulsive behavior. Nat Med. 2021;27:232–8.
Loh A, Gwun D, Chow CT, Boutet A, Tasserie J, Germann J, et al. Probing responses to deep brain stimulation with functional magnetic resonance imaging. Brain Stimul. 2022;15:683–94.
Gonzalez-Escamilla G, Muthuraman M, Ciolac D, Coenen VA, Schnitzler A, Groppa S. Neuroimaging and electrophysiology meet invasive neurostimulation for causal interrogations and modulations of brain states. Neuroimage. 2020;220:117144.
Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7.
Cha J, Choi KS, Rajendra JK, McGrath CL, Riva-Posse P, Holtzheimer PE, et al. Whole brain network effects of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02306-6.
Figee M, Luigjes J, Smolders R, Valencia-Alfonso C-E, Van Wingen G, De Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013;16:386–7.
Fridgeirsson EA, Figee M, Luigjes J, van den Munckhof P, Schuurman PR, van Wingen G, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive-compulsive disorder. Brain. 2020;143:1603–12.
Elias GJB, Germann J, Boutet A, Loh A, Li B, Pancholi A, et al. 3 T MRI of rapid brain activity changes driven by subcallosal cingulate deep brain stimulation. Brain. 2021;145:2214–26.
Deng Y, Li W, Zhang B. Functional activity in the effect of transcranial magnetic stimulation therapy for patients with depression: a meta-analysis. J Pers Med. 2023;13:405.
Zheng A, Yu R, Du W, Liu H, Zhang Z, Xu Z, et al. Two-week rTMS-induced neuroimaging changes measured with fMRI in depression. J Affect Disord. 2020;270:15–21.
Scherer M, Harmsen IE, Samuel N, Elias GJB, Germann J, Boutet A, et al. Oscillatory network markers of subcallosal cingulate deep brain stimulation for depression. Brain Stimul. 2023;16:1764–75.
Waters AC, Veerakumar A, Choi KS, Howell B, Tiruvadi V, Bijanki KR, et al. Test-retest reliability of a stimulation-locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp. 2018;39:4844–56.
Jbabdi S, Lehman JF, Haber SN, Behrens TE. Human and monkey ventral prefrontal fibers use the same organizational principles to reach their targets: tracing versus tractography. J Neurosci. 2013;33:3190–201.
Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW, et al. Deep brain stimulation: current challenges and future directions. Nat Rev Neurol. 2019;15:148–60.
Ashkan K, Rogers P, Bergman H, Ughratdar I. Insights into the mechanisms of deep brain stimulation. Nat Rev Neurol. 2017;13:548–54.
Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304.
Lazari A, Salvan P, Cottaar M, Papp D, Rushworth MFS, Johansen-Berg H. Hebbian activity-dependent plasticity in white matter. Cell Rep. 2022;39:110951.
van den Boom BJG, Elhazaz-Fernandez A, Rasmussen PA, van Beest EH, Parthasarathy A, Denys D, et al. Unraveling the mechanisms of deep-brain stimulation of the internal capsule in a mouse model. Nat Commun. 2023;14:5385.
Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608.
Passingham RE, Wise SP. The neurobiology of the prefrontal cortex: anatomy, evolution, and the origin of insight. 1st ed. Oxford University Press, USA; 2012.
Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86.
Pagani M, Gutierrez-Barragan D, de Guzman AE, Xu T, Gozzi A. Mapping and comparing fMRI connectivity networks across species. Commun Biol. 2023;6:1238.
Lehman JF, Greenberg BD, McIntyre CC, Rasmussen SA, Haber SN. Rules ventral prefrontal cortical axons use to reach their targets: implications for diffusion tensor imaging tractography and deep brain stimulation for psychiatric illness. J Neurosci. 2011;31:10392–402.
Safadi Z, Grisot G, Jbabdi S, Behrens TE, Heilbronner SR, McLaughlin NCR, et al. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci. 2018;38:2106–17.
Baldermann JC, Melzer C, Zapf A, Kohl S, Timmermann L, Tittgemeyer M, et al. Connectivity profile predictive of effective deep brain stimulation in obsessive-compulsive disorder. Biol Psychiatry. 2019;85:735–43.
Schmid MC, Mrowka SW, Turchi J, Saunders RC, Wilke M, Peters AJ, et al. Blindsight depends on the lateral geniculate nucleus. Nature. 2010;466:373–7.
Murray EA, Moylan EJ, Saleem KS, Basile BM, Turchi J. Specialized areas for value updating and goal selection in the primate orbitofrontal cortex. Elife. 2015;4:e11695.
Amiez C, Joseph JP, Procyk E. Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16:1040–55.
Rajalingham R, DiCarlo JJ. Reversible inactivation of different millimeter-scale regions of primate IT results in different patterns of core object recognition deficits. Neuron. 2019;102:493–505.e5.
Wang JB, Aryal M, Zhong Q, Vyas DB, Airan RD. Noninvasive ultrasonic drug uncaging maps whole-brain functional networks. Neuron. 2018;100:728–38.e7.
Perolina E, Meissner S, Raos B, Harland B, Thakur S, Svirskis D. Translating ultrasound-mediated drug delivery technologies for CNS applications. Adv Drug Deliv Rev. 2024;208:115274.
Wasielewska JM, White AR. Focused ultrasound-mediated drug delivery in humans - a path towards translation in neurodegenerative diseases. Pharm Res. 2022;39:427–39.
Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–97.
Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–63.
Berry AS, Harrison TM. New perspectives on the basal forebrain cholinergic system in Alzheimer’s disease. Neurosci Biobehav Rev. 2023;150:105192.
Pople CB, Meng Y, Li DZ, Bigioni L, Davidson B, Vecchio LM, et al. Neuromodulation in the treatment of alzheimer’s disease: current and emerging approaches. J Alzheimers Dis. 2020;78:1299–313.
Turchi J, Chang C, Ye FQ, Russ BE, Yu DK, Cortes CR, et al. The basal forebrain regulates global resting-state fMRI fluctuations. Neuron. 2018;97:940–952.e4.
Mesulam MM, Van Hoesen GW. Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res. 1976;109:152–7.
Alexander L, Gaskin PLR, Sawiak SJ, Fryer TD, Hong YT, Cockcroft GJ, et al. Fractionating blunted reward processing characteristic of anhedonia by over-activating primate subgenual anterior cingulate cortex. Neuron. 2019;101:307–20.e6.
Anderson IM. Selective serotonin reuptake inhibitors versus tricyclic antidepressants: a meta-analysis of efficacy and tolerability. J Affect Disord. 2000;58:19–36.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104:5163–8.
Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, et al. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–97.
Krut’ VG, Kalinichenko AL, Maltsev DI, Jappy D, Shevchenko EK, Podgorny OV, et al. Optogenetic and chemogenetic approaches for modeling neurological disorders in vivo. Prog Neurobiol. 2024;235:102600.
Dai J, Brooks DI, Sheinberg DL. Optogenetic and electrical microstimulation systematically bias visuospatial choice in primates. Curr Biol. 2014;24:63–69.
Ohayon S, Grimaldi P, Schweers N, Tsao DY. Saccade modulation by optical and electrical stimulation in the macaque frontal eye field. J Neurosci. 2013;33:16684–97.
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–9.
Gerits A, Vanduffel W. Optogenetics in primates: a shining future? Trends Genet. 2013;29:403–11.
Touroutoglou A, Bliss-Moreau E, Zhang J, Mantini D, Vanduffel W, Dickerson BC, et al. A ventral salience network in the macaque brain. Neuroimage. 2016;132:190–7.
Gerits A, Farivar R, Rosen BR, Wald LL, Boyden ES, Vanduffel W. Optogenetically induced behavioral and functional network changes in primates. Curr Biol. 2012;22:1722–6.
Ortiz-Rios M, Agayby B, Balezeau F, Haag M, Rima S, Cadena-Valencia J, et al. Optogenetic stimulation of the primary visual cortex drives activity in the visual association cortex. Curr Res Neurobiol. 2023;4:100087.
Song J, Patel RV, Sharif M, Ashokan A, Michaelides M. Chemogenetics as a neuromodulatory approach to treating neuropsychiatric diseases and disorders. Mol Ther. 2022;30:990–1005.
Roseboom PH, Mueller SAL, Oler JA, Fox AS, Riedel MK, Elam VR, et al. Evidence in primates supporting the use of chemogenetics for the treatment of human refractory neuropsychiatric disorders. Mol Ther. 2021;29:3484–97.
Raper J, Galvan A. Applications of chemogenetics in non-human primates. Curr Opin Pharm. 2022;64:102204.
Nagai Y, Miyakawa N, Takuwa H, Hori Y, Oyama K, Ji B, et al. Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys. Nat Neurosci. 2020;23:1157–67.
Oyama K, Hori Y, Nagai Y, Miyakawa N, Mimura K, Hirabayashi T, et al. Chronic behavioral manipulation via orally delivered chemogenetic actuator in macaques. J Neurosci. 2022;42:2552–61.
Nagai Y, Kikuchi E, Lerchner W, Inoue K-I, Ji B, Eldridge MAG, et al. PET imaging-guided chemogenetic silencing reveals a critical role of primate rostromedial caudate in reward evaluation. Nat Commun. 2016;7:13605.
Kimura K, Nagai Y, Hatanaka G, Fang Y, Tanabe S, Zheng A, et al. A mosaic adeno-associated virus vector as a versatile tool that exhibits high levels of transgene expression and neuron specificity in primate brain. Nat Commun. 2023;14:4762.
Upright NA, Brookshire SW, Schnebelen W, Damatac CG, Hof PR, Browning PGF, et al. Behavioral effect of chemogenetic inhibition is directly related to receptor transduction levels in Rhesus Monkeys. J Neurosci. 2018;38:7969–75.
Yan X, Telu S, Dick RM, Liow J-S, Zanotti-Fregonara P, Morse CL, et al. [11C]deschloroclozapine is an improved PET radioligand for quantifying a human muscarinic DREADD expressed in monkey brain. J Cereb Blood Flow Metab. 2021;41:2571–82.
Fujimoto A, Elorette C, Fredericks JM, Fujimoto SH, Fleysher L, Rudebeck PH, et al. Resting-state fMRI-based screening of deschloroclozapine in Rhesus Macaques predicts dosage-dependent behavioral effects. J Neurosci. 2022;42:5705–16.
Wood CM, Alexander L, Alsiö J, Santangelo AM, McIver L, Cockcroft GJ, et al. Chemogenetics identifies separate area 25 brain circuits involved in anhedonia and anxiety in marmosets. Sci Transl Med. 2023;15:eade1779.
Miyakawa N, Nagai Y, Hori Y, Mimura K, Orihara A, Oyama K, et al. Chemogenetic attenuation of cortical seizures in nonhuman primates. Nat Commun. 2023;14:971.
Elorette C, Fujimoto A, Stoll FM, Fujimoto SH, Fleysher L, Bienkowska N, et al. The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation. Nat Commun. 2024;15:4669.
Zeisler ZR, London L, Janssen WG, Fredericks JM, Elorette C, Fujimoto A, et al. Single basolateral amygdala neurons in macaques exhibit distinct connectional motifs with frontal cortex. Neuron. 2023;111:3307–3320.e5.
Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23.
Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80.
Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28.
Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118.
Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–61.
Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163.
Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry Residents’ J. 2020;16:6–6.
Benabid AL. Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol. 2003;13:696–706.
Min H-K, Ross EK, Jo HJ, Cho S, Settell ML, Jeong JH, et al. Dopamine release in the nonhuman primate caudate and putamen depends upon site of stimulation in the subthalamic nucleus. J Neurosci. 2016;36:6022–9.
Min H-K, Ross EK, Lee KH, Dennis K, Han SR, Jeong JH, et al. Subthalamic nucleus deep brain stimulation induces motor network BOLD activation: use of a high precision MRI guided stereotactic system for nonhuman primates. Brain Stimul. 2014;7:603–7.
Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009;12:1370–1.
Fujimoto SH, Fujimoto A, Elorette C, Seltzer A, Andraka E, Verma G, et al. Deep brain stimulation induces white matter remodeling and functional changes to brain-wide networks. bioRxiv. 2024. 2024.06.13.598710
Kadono Y, Koguchi K, Okada K-I, Hosomi K, Hiraishi M, Ueguchi T, et al. Repetitive transcranial magnetic stimulation restores altered functional connectivity of central poststroke pain model monkeys. Sci Rep. 2021;11:6126.
Ohnishi T, Hayashi T, Okabe S, Nonaka I, Matsuda H, Iida H, et al. Endogenous dopamine release induced by repetitive transcranial magnetic stimulation over the primary motor cortex: an [11C]raclopride positron emission tomography study in anesthetized macaque monkeys. Biol Psychiatry. 2004;55:484–9.
Salinas FS, Szabó CÁ, Zhang W, Jones L, Leland MM, Wey H-Y, et al. Functional neuroimaging of the baboon during concurrent image-guided transcranial magnetic stimulation. Neuroimage. 2011;57:1393–401.
O’Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MFS. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54:479–90.
Perera ND, Alekseichuk I, Shirinpour S, Wischnewski M, Linn G, Masiello K, et al. Dissociation of centrally and peripherally induced transcranial magnetic stimulation effects in nonhuman primates. J Neurosci. 2023;43:8649–62.
Chen H, Felix C, Folloni D, Verhagen L, Sallet J, Jerusalem A. Modelling transcranial ultrasound neuromodulation: an energy-based multiscale framework. Acta Biomater. 2022;151:317–32.
Munoz F, Aurup C, Konofagou EE, Ferrera VP. Modulation of brain function and behavior by focused ultrasound. Curr Behav Neurosci Rep. 2018;5:153–64.
Verhagen L, Gallea C, Folloni D, Constans C, Jensen DE, Ahnine H, et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife. 2019;8:e40541.
Folloni D, Verhagen L, Mars RB, Fouragnan E, Constans C, Aubry J-F, et al. Manipulation of subcortical and deep cortical activity in the primate brain using transcranial focused ultrasound stimulation. Neuron. 2019;101:1109–1116.e5.
Liu D, Munoz F, Sanatkhani S, Pouliopoulos AN, Konofagou EE, Grinband J, et al. Alteration of functional connectivity in the cortex and major brain networks of non-human primates following focused ultrasound exposure in the dorsal striatum. Brain Stimul. 2023;16:1196–204.
Monosov IE, Zimmermann J, Frank MJ, Mathis MW, Baker JT. Ethological computational psychiatry: challenges and opportunities. Curr Opin Neurobiol. 2024;86:102881.
Young ME, Spencer-Salmon, Mosher C, Tamang S, Rajan K, Rudebeck PH. Temporally-specific sequences of neural activity in interconnected corticolimbic structures during reward anticipation. Neuron. 2023;111:3668–3682.e5.
Acknowledgements
The authors would like to thank their funders and other members of the PR, BR, and HM labs for advice and encouragement.
Funding
SF, KSC, BR, HM, and PR are supported by the grant from Hope for Depression Research Foundation and the grant from The National Institute of Mental Health (NIMH) (R01MH132789). SF, AF, CE, BR, and PR are supported by grants from NIMH and the BRAIN initiative (R01MH110822 and RF1MH117040). BR is supported by grants from NIMH (R01MH111439) and NINDS (R01NS109498). AF is supported by Overseas Research Fellowship from Takeda Science Foundation and a Brain & Behavior Research Foundation Young Investigator grant (#28979).
Author information
Authors and Affiliations
Contributions
All authors wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
HM and KSC receive consulting fees from Abbott Neuromodulation. Other authors declare no competing financial interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujimoto, S., Fujimoto, A., Elorette, C. et al. What can neuroimaging of neuromodulation reveal about the basis of circuit therapies for psychiatry?. Neuropsychopharmacol. (2024). https://doi.org/10.1038/s41386-024-01976-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-024-01976-2