Abstract
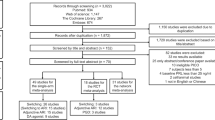
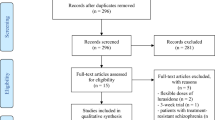
Antipsychotic-induced sialorrhea carries a significant burden, but evidence-based treatment guidance is incomplete, warranting network meta-analysis (NMA) of pharmacological interventions for antipsychotic-related sialorrhea. PubMed Central/PsycInfo/Cochrane Central database/Clinicaltrials.gov/WHO-ICTRP and the Chinese Electronic Journal Database (Qikan.cqvip.com) were searched for published/unpublished RCTs of antipsychotic-induced sialorrhea (any definition) in adults, up to 06/12/2023. We assessed global/local inconsistencies, publication bias, risk of bias (RoB2), and confidence in the evidence, conducting subgroup/sensitivity analyses. Co-primary efficacy outcomes were changes in saliva production (standardized mean difference/SMD) and study-defined response (risk ratios/RRs). The acceptability outcome was all-cause discontinuation (RR). Primary nodes were molecules; the mechanism of action (MoA) was secondary. Thirty-four RCTs entered a systematic review, 33 NMA (n = 1958). All interventions were for clozapine-induced sialorrhea in subjects with mental disorders. Regarding individual agents and response, metoclopramide (RR = 3.11, 95% C.I. = 1.39–6.98), cyproheptadine, (RR = 2.76, 95% C.I. = 2.00–3.82), sulpiride (RR = 2.49, 95% C.I. = 1.65–3.77), propantheline (RR = 2.39, 95% C.I. = 1.97–2.90), diphenhydramine (RR = 2.32, 95% C.I. = 1.88–2.86), benzhexol (RR = 2.32, 95% C.I. = 1.59–3.38), doxepin (RR = 2.30, 95% C.I. = 1.85–2.88), amisulpride (RR = 2.23, 95% C.I. = 1.30–3.81), chlorpheniramine (RR = 2.20, 95% C.I. = 1.67–2.89), amitriptyline (RR = 2.09, 95% C.I. = 1.34–3.26), atropine, (RR = 2.03, 95% C.I. = 1.22–3.38), and astemizole, (RR = 1.70, 95% C.I. = 1.28–2.26) outperformed placebo, but not glycopyrrolate or ipratropium. Across secondary nodes (k = 28, n = 1821), antimuscarinics (RR = 2.26, 95% C.I. = 1.91–2.68), benzamides (RR = 2.23, 95% C.I. = 1.75–3.10), TCAs (RR = 2.23, 95% C.I. = 1.83–2.72), and antihistamines (RR = 2.18, 95% C.I. = 1.83–2.59) outperformed placebo. In head-to-head comparisons, astemizole and ipratropium were outperformed by several interventions. All secondary nodes, except benzamides, outperformed the placebo on the continuous efficacy outcome. For nocturnal sialorrhea, neither benzamides nor atropine outperformed the placebo. Active interventions did not differ significantly from placebo regarding constipation or sleepiness/drowsiness. Low-confidence findings prompt caution in the interpretation of the results. Considering primary nodes’ co-primary efficacy outcomes and head-to-head comparisons, efficacy for sialorrhea is most consistent for the following agents, decreasing from metoclopramide through cyproheptadine, sulpiride, propantheline, diphenhydramine, benzhexol, doxepin, amisulpride, chlorpheniramine, to amitriptyline, and atropine (the latter not for nocturnal sialorrhea). Shared decision-making with the patient should guide treatment decisions regarding clozapine-related sialorrhea.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
With the publication of this article, the entire dataset will be available to the final reader upon request. All authors had access to the data and were responsible for the decision to submit it for publication.
References
Mohandoss AA, Thavarajah R. Salivary flow alteration in patients undergoing treatment for schizophrenia: disease-drug-target gene/protein association study for side-effects. J Oral Biol Craniofac Res. 2019;9:286–93.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51.
Kane JM, Durgam S, Satlin A, Vanover KE, Chen R, Davis R, et al. Safety and tolerability of lumateperone for the treatment of schizophrenia: a pooled analysis of late-phase placebo- and active-controlled clinical trials. Int Clin Psychopharmacol. 2021;36:244–50.
Carbon M, Hsieh CH, Kane JM, Correll CU. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78:e264–e278.
Carbon M, Kane JM, Leucht S, Correll CU. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17:330–40.
Wagner E, Siafis S, Fernando P, Falkai P, Honer WG, Röh A, et al. Efficacy and safety of clozapine in psychotic disorders—a systematic quantitative meta-review. Transl Psychiatry. 2021;11:487.
Citrome L, McEvoy JP, Saklad SR. Guide to the management of clozapine-related tolerability and safety concerns. Clin Schizophr Relat Psychoses. 2016;10:163–77.
Gupta S, Khastgir U, Croft M, Roshny S. Management of clozapine-induced sialorrhoea. BJPsych Adv. 2020;26:106–8.
Chen SY, Ravindran G, Zhang Q, Kisely S, Siskind D. Treatment strategies for clozapine-induced sialorrhea: a systematic review and meta-analysis. CNS Drugs. 2019;33:225–38.
Syed R, Au K, Cahill C, Duggan L, He Y, Udu V, et al. Pharmacological interventions for clozapine-induced hypersalivation. Cochrane Database Syst Rev. 2008; Cd005579.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–11.
Mavridis D. Network meta-analysis in a nutshell. Evid Based Ment Health. 2019;22:100–1.
Chengappa KR, Pollock BG, Parepally H, Levine J, Kirshner MA, Brar JS, et al. Anticholinergic differences among patients receiving standard clinical doses of olanzapine or clozapine. J Clin Psychopharmacol. 2000;20:311–6.
Halder A, Ravindran NP, Nagda P, Harshe D, Harshe G. Review of psychotropic agents associated with Sialorrhoea, except Clozapine. Indian J Psychol Med. 2021;45:14–18.
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/. 2021, Accessed Date 2021.
Qurashi I, Chu S, Husain N, Drake RJ, Chaudhry I, Deakin J. Glycopyrrolate in comparison to hyoscine hydrobromide and placebo in the treatment of hypersalivation induced by clozapine (GOTHIC1): study protocol for a randomised controlled feasibility study. Trials. 2016;17:1–9.
Steinlechner S, Klein C, Moser A, Lencer R, Hagenah J. Botulinum toxin B as an effective and safe treatment for neuroleptic-induced sialorrhea. Psychopharmacology. 2010;207:593–7.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Papakonstantinou T, Nikolakopoulou A, Higgins JP, Egger M, Salanti G. Cinema: software for semiautomated assessment of the confidence in the results of network meta‐analysis. Campbell Syst Rev. 2020;16:e1080.
Balduzzi S, Rücker G, Nikolakopoulou A, Papakonstantinou T, Salanti G, Efthimiou O, et al. netmeta: An R Package for network meta-analysis using frequentist methods. J Stat Softw. 2023;106:1–40.
Team R. RStudio: Integrated development for R. PBC: Boston, MA, 2020.
Higgins J, Jackson D, Barrett J, Lu G, Ades A, White I. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods. 2012;3:98–110.
Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332–45.
Salanti G, Ades A, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71.
Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Res Synth Methods. 2012;3:161–76.
Sockalingam S, Shammi C, Remington G. Treatment of clozapine-induced hypersalivation with ipratropium bromide: a randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2009;70:1114–9.
Kreinin A, Miodownik C, Mirkin V, Gaiduk Y, Yankovsky Y, Bersudsky Y, et al. Double-blind, randomized, placebo-controlled trial of metoclopramide for hypersalivation associated with clozapine. J Clin Psychopharmacol. 2016;36:200–5.
Mubaslat O, Lambert T. The effect of sublingual atropine sulfate on clozapine-induced hypersalivation: a multicentre, randomised placebo-controlled trial. Psychopharmacol (Berl). 2020;237:2905–15.
Segev A, Evans A, Hodsoll J, Whiskey E, Sheriff RS, Shergill S, et al. Hyoscine for clozapine-induced hypersalivation: a double-blind, randomized, placebo-controlled cross-over trial. Int Clin Psychopharmacol. 2019;34:101–7.
Li Z, Song Z, Cheng P, Li C, Xiang X, Shi H. A double-blinded controlled trial on the use of astemizole in clozapine-induced sialorrhoea. Chin J Clin Psychiatry. 1993;03:34–35.
Lu X, Xue S, Yang M. The use of diphehydramine in clozapine-induced sialorrhoea. Sichuan J Ment Health. 1998;11:38–40.
Lu L, Liu G, Zhao J, Liu A, Liu S, Liu L, et al. A double blinded placebo-controlled trial on the use of chlorpheniramine in clozapine-induced sialorrhoea. Chin J Neurol. 1994;27:252–3.
Yao H, Qu H, Li G. A double-blind controlled study of propantheline and astemizole in the treatment of salivation side effects of clozapine. Chin J Clin Psychiatry. 1994;1:4.
Fan Z, Zheng C, Li S. placebo-controlled trial on the use of propantheline in clozapine-induced sialorrhoea. Strait Pharm J. 1996;8:33–34.
Yang S, Xu S, Yuhua Y. Double-blind control of cyproheptadine and placebo in the treatment of side effects of clozapine-induced salivation. J Chin People’s Health. 1996;8:12–13.
Gong G, Yuan L, Qin D, Li H, Zhao L, Yang X, et al. A double-blind controlled study of diphenhydramine, propantheline, astemizole and placebo in the treatment of clozapine-induced sialorrhea. Chin J Psychiatry. 1998;31:138.
Xu H, Dong A, Zhang T, Shu W. Different doses of diphenhydramine in the treatment of clozapine-induced salivation. N. Drugs Clin Rem. 1997;16:205–7.
Yang H, Huang S, Feng C, Chen J. Study on the treatment of salivation induced by clozapine. J Binzhou Med Univ. 1999;22:451.
Tao J, Yin X, Liu J. A randomized controlled study of sulpiride in the treatment of clozapine-induced salivation. J Neurosci Ment Health. 2007;7:141–3.
Zhang Z, Sun T. Comparative observation of the effect of diphenhydramine and doxepin in treatment of salivation caused by clozapine. J Huaihai Med. 1999;17:15–16.
Zhou Y. Clinical observation of chlorpheniramine in the treatment of salivation caused by clozapine. Linchuang jingshen yixue zazhi. J Clin Psychiatry. 2009;19:132–3.
Zhou H, Zhang Q. A double-blind controlled study of doxepin and propantheline in the treatment of clozapine-induced hypersalivation. Linchuang jingshen yixue zazhi. J Clin Psychiatry. 1996;6:300.
Yang M, Xue S, Wen Q. A double-blind controlled study of diphenhydramine and propantheline in the treatment of salivation side effects of clozapine. Guangdong Med J. 1997;18:731.
Wang S, Song W, Gu W. Comparison of doxepin and astemizole in the treatment of clozapine-induced salivation. Shanghai Arch Psychiatry. 1998;10:176–7.
Song Y, Feng X, Lv Y, Zhang S. A comparative study of doxepin and propantheline in the treatment of clozapine-induced sialorrhea. Shandong Arch Psychiatry. 1996;9:17–9.
Lin H. Double-blind comparison of propantheline and placebo in the treatment of salivation side effects of clozapine. Chin J Neuropsychiatr Dis. 1999;25:316–7.
Li D, Xu M, Duan X. A comparative observation of cyproheptadine and propantheline in the treatment of clozapine-induced salivation. Occup Health. 2003;19:176–7.
Man WH, Colen-de Koning JC, Schulte PF, Cahn W, van Haelst IM, Doodeman HJ, et al. The effect of glycopyrrolate on nocturnal sialorrhea in patients using clozapine: a randomized, crossover, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2017;37:155–61.
Li Z, Wang Q. A controlled study of amisulpride in the treatment of clozapine-induced sialorrhea. World’s Latest Med Inf Dig. 2015;88:111.
Li C. Observation on the efficacy of promethazine in the treatment of adverse reactions of clozapine salivation. Chin Manip Rehabilit Med. 2011;54:115.
Qin J, Cui C, Ge F. A comparison of treatments for clozapine-induced sialorrhea. Chin J Clin Psychiatry. 1999;9:225–6.
Yu G, Ding G, Liang S. Treatment of clozapine-induced sialorrhea. Chin J Clin Psychiatry. 2000;10:288.
Cheng J, Jiang S. A study on the use of benzhexol in the treatment of clozapine-induced sialorrhea. Mod J Integr Tradit Chin West Med. 2005;14:2135–6.
Wang J, Ning Y, Wang W, Yu J. A double-blind controlled study of cyproheptadine and benzhexol in the treatment of clozapine-induced sialorrhea. Chin J Neuropsychiatr Dis. 2000;26:111–2.
Kreinin A, Novitski D, Weizman A. Amisulpride treatment of clozapine-induced hypersalivation in schizophrenia patients: a randomized, double-blind, placebo-controlled cross-over study. Int Clin Psychopharmacol. 2006;21:99–103.
Bai YM, Lin CC, Chen JY, Liu WC. Therapeutic effect of pirenzepine for clozapine-induced hypersalivation: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Psychopharmacol. 2001;21:608–11.
Zhao Z, Chen H, Xie B, Sun S, Wu H, Ma Q, et al. A controlled trial on the use of Zhiyan capsule in clozapine-induced sialorrhoea. Chin J Civ Aff Med. 2000;12:95–6.
Liang CS, Ho PS, Shen LJ, Lee WK, Yang FW, Chiang KT. Comparison of the efficacy and impact on cognition of glycopyrrolate and biperiden for clozapine-induced sialorrhea in schizophrenic patients: a randomized, double-blind, crossover study. Schizophr Res. 2010;119:138–44.
Sridharan K, Sivaramakrishnan G. Pharmacological interventions for treating sialorrhea associated with neurological disorders: a mixed treatment network meta-analysis of randomized controlled trials. J Clin Neurosci. 2018;51:12–7.
Proctor GB, Carpenter GH. Salivary secretion: mechanism and neural regulation. Saliva: Secret Funct. 2014;24:14–29.
Proctor GB. The physiology of salivary secretion. Periodontology 2000. 2016;70:11–25.
Zorn SH, Jones SB, Ward KM, Liston DR. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol: Mol Pharmacol. 1994;269:R1–2.
Ryberg AT, Warfvinge G, Axelsson L, Soukup O, Götrick B, Tobin G. Expression of muscarinic receptor subtypes in salivary glands of rats, sheep and man. Arch Oral Biol. 2008;53:66–74.
Godoy T, Riva A, Ekström J. Clozapine‐induced salivation: interaction with N‐desmethylclozapine and amisulpride in an experimental rat model. Eur J oral Sci. 2011;119:275–81.
Olianas MC, Maullu C, Onali P. Mixed agonist-antagonist properties of clozapine at different human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary cells. Neuropsychopharmacology. 1999;20:263–70.
Sur C, Mallorga PJ, Wittmann M, Jacobson MA, Pascarella D, Williams JB, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc Natl Acad Sci. 2003;100:13674–9.
Ekström J, Godoy T, Riva A. Clozapine: agonistic and antagonistic salivary secretory actions. J Dent Res. 2010;89:276–80.
Lamba G, Ellison JM. Reducing clozapine-induced hypersalivation. Curr Psychiatry. 2011;10:77–9.
Sato T, Yajima T, Fujita M, Kobashi M, Ichikawa H, Yoshida R, et al. Orexin A and B in the rat superior salivatory nucleus. Autonomic Neurosci. 2020;228:102712.
Linehan V, Trask RB, Briggs C, Rowe TM, Hirasawa M. Concentration-dependent activation of dopamine receptors differentially modulates GABA release onto orexin neurons. Eur J Neurosci. 2015;42:1976–83.
Mizuchi A, Kitagawa N, Miyachi Y. Regional distribution of sultopride and sulpiride in rat brain measured by radioimmunoassay. Psychopharmacol (Berl). 1983;81:195–8.
Abbas AI, Hedlund PB, Huang XP, Tran TB, Meltzer HY, Roth BL. Amisulpride is a potent 5-HT7 antagonist: relevance for antidepressant actions in vivo. Psychopharmacol (Berl). 2009;205:119–28.
Bourdon DM, Camden JM, Landon LA, Levy FO, Turner JT. Identification of the adenylyl cyclase-activating 5-hydroxytryptamine receptor subtypes expressed in the rat submandibular gland. Br J Pharm. 2000;130:104–8.
Stanton T, Bolden-Watson C, Cusack B, Richelson E. Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics. Biochem Pharmacol. 1993;45:2352–4.
Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. J Clin Epidemiol. 2004;57:1124–30.
Acknowledgements
The authors sincerely acknowledge Dr. Giulia Chock, MD, for her assistance in the appraisal of the Chinese language evidence at review.
Author information
Authors and Affiliations
Contributions
MF, CC, and MS conceived the study. CUC and AdB coordinated the study. NS, MDP, MB, MB, RS, SC, ML, DS, GP, AB and FS assisted in data extraction, analysis, and interpretation. MF and CC wrote the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
CUC reported receiving personal fees from Alkermes plc, Allergan plc, Angelini Pharma, Gedeon Richter, Gerson Lehrman Group, Intra-Cellular Therapies, Inc, Janssen Pharmaceutica/Johnson&Johnson, LB Pharma International BV,H Lundbeck A/S, MedAvante-ProPhase, Medscape, Neurocrine Biosciences, Noven Pharmaceuticals, Inc, Otsuka Pharmaceutical Co, Inc, Pfizer, Inc, Recordati, Rovi, Sumitomo Dainippon Pharma, Sunovion Pharmaceuticals, Inc, Supernus Pharmaceuticals, Inc, Takeda Pharmaceutical Company Limited, Teva Pharmaceuticals, Acadia Pharmaceuticals, Inc, Axsome Therapeutics, Inc, Indivior, Merck&Co, Mylan NV, MedInCell, and Karuna Therapeutics and grants from Janssen Pharmaceutica, Takeda Pharmaceutical Company Limited, Berlin Institute of Health, the National Institute of Mental Health, Patient Centered Outcomes Research Institute, and the Thrasher Foundation outside the submitted work; receiving royalties from UpToDate; and holding stock options in LB Pharma. No other disclosures were reported. AdB has received research support from Janssen, Lundbeck, and Otsuka and lecture fees for educational meeting from Chiesi, Lundbeck, Roche, Sunovion, Viatris, Recordati, Angelini and Takeda; he has served on advisory boards for Eli Lilly, Jansen, Lundbeck, Otsuka, Roche, and Takeda, Chiesi, Recordati, Angelini, Vitria. MS has received honoraria/has been a consultant for Angelini, Lundbeck, and Otsuka. MF received honoraria from the American Society of Clinical Psychopharmacology (ASCP), Angelini, Lundbeck, Bristol Meyer Squibb, and Boehringer-Ingelheim for his speaker activities. GP has received research grants and/or has been a consultant for, and/or has been a speaker for: Angelini, Lundbeck, Janssen, Otsuka, Pfizer, Roche, Eisai. DS is funded in part by an NHMRC EL2 Fellowship GNT1194635. FS has received honoraria/has been a consultant for Janssen (past five years). The other authors report no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fornaro, M., Caiazza, C., Solini, N. et al. Pharmacological interventions for antipsychotic-related sialorrhea: a systematic review and network meta-analysis of randomized trials. Mol Psychiatry 28, 3648–3660 (2023). https://doi.org/10.1038/s41380-023-02266-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02266-x