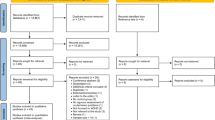
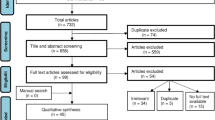
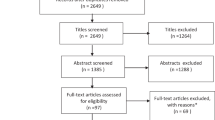
Abstract
Aim
To conduct a systematic review and meta-analysis assessing whether vision and/or eye disorders are associated with Autism Spectrum Disorder (ASD).
Method
Based on a pre-registered protocol (PROSPERO: CRD42022328485), we searched PubMed, Web of Knowledge/Science, Ovid Medline, Embase and APA PsycINFO up to 5th February 2022, with no language/type of document restrictions. We included observational studies 1) reporting at least one measure of vision in people of any age with a diagnosis of ASD based on DSM or ICD criteria, or ADOS; or 2) reporting the prevalence of ASD in people with and without vision disorders. Study quality was assessed with the Appraisal tool for Cross-Sectional Studies (AXIS). Random-effects meta-analyses were used for data synthesis.
Results
We included 49 studies in the narrative synthesis and 46 studies in the meta-analyses (15,629,159 individuals distributed across multiple different measures). We found meta-analytic evidence of increased prevalence of strabismus (OR = 4.72 [95% CI: 4.60, 4.85]) in people with versus those without ASD (non-significant heterogeneity: Q = 1.0545, p = 0.7881). We also found evidence of increased accommodation deficits (Hedge’s g = 0.68 [CI: 0.28, 1.08]) (non-significant heterogeneity: Q = 6.9331, p = 0.0741), reduced peripheral vision (−0.82 [CI: −1.32, −0.33]) (non-significant heterogeneity: Q = 4.8075, p = 0.4398), reduced stereoacuity (0.73 [CI: −1.14, −0.31]) (non-significant heterogeneity: Q = 0.8974, p = 0.3435), increased color discrimination difficulties (0.69 [CI: 0.27,1.10]) (non-significant heterogeneity: Q = 9.9928, p = 0.1890), reduced contrast sensitivity (0.45 [CI: −0.60, −0.30]) (non-significant heterogeneity: Q = 9.9928, p = 0.1890) and increased retinal thickness (=0.29 [CI: 0.07, 0.51]) (non-significant heterogeneity: Q = 0.8113, p = 0.9918) in ASD.
Discussion
ASD is associated with some self-reported and objectively measured functional vision problems, and structural alterations of the eye, even though we observed several methodological limitations in the individual studies included in our meta-analyses. Further research should clarify the causal relationship, if any, between ASD and problems of vision during early life.
PROSPERO registration
CRD42022328485.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Raw data and R codes for the meta-analysis are available upon request to the corresponding author.
Change history
09 August 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41380-023-02212-x
References
Solmi M, Song M, Yon DK, Lee SW, Fombonne E, Kim MS, et al. Incidence, prevalence, and global burden of autism spectrum disorder from 1990 to 2019 across 204 countries. Mol Psychiatry. 2022;27:4172–80.
Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association; 2013.
Leekam SR, Nieto C, Libby SJ, Wing L, Gould J. Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord. 2007;37:894–910.
Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory Experiences Questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006;47:591–601.
Parmar KR, Porter CS, Dickinson CM, Pelham J, Baimbridge P, Gowen E. Visual sensory experiences from the viewpoint of autistic adults. Front Psychol. 2021;12:633037.
Butchart M, Long JJ, Brown M, McMillan A, Bain J, Karatzias T. Autism and visual impairment: a review of the literature. Rev J Autism Dev Disorders. 2017;4:118–31.
Wrzesinska M, Kapias J, Nowakowska-Domagala K, Kocur J. Visual impairment and traits of autism in children. Psychiatr Pol. 2017;51:349–58.
Do B, Lynch P, Macris EM, Smyth B, Stavrinakis S, Quinn S, et al. Systematic review and meta-analysis of the association of Autism Spectrum Disorder in visually or hearing impaired children. Ophthalmic Physiol Opt. 2017;37:212–24.
Leung MP, Thompson B, Black J, Dai S, Alsweiler JM. The effects of preterm birth on visual development. Clin Exp Optom. 2018;101:4–12.
Lynn WA, Lightman S. The eye in systemic infection. Lancet. 2004;364:1439–50.
Whatham A, Bartlett H, Eperjesi F, Blumenthal C, Allen J, Suttle C, et al. Vitamin and mineral deficiencies in the developed world and their effect on the eye and vision. Ophthalmic Physiol Opt. 2008;28:1–12.
Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8:13.
Grabrucker AM. Environmental factors in autism. Front Psychiatry. 2012;3:118.
Al-Haddad BJS, Oler E, Armistead B, Elsayed NA, Weinberger DR, Bernier R, et al. The fetal origins of mental illness. Am J Obstet Gynecol. 2019;221:549–62.
Strasser L, Downes M, Kung J, Cross JH, De Haan M. Prevalence and risk factors for autism spectrum disorder in epilepsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2018;60:19–29.
Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–16.
London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53.
Ecker C. The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical setting. Autism. 2017;21:18–28.
Kelley KW, Pașca SP. Human brain organogenesis: toward a cellular understanding of development and disease. Cell. 2022;185:42–61.
MacCormick IJ, Czanner G, Faragher B. Developing retinal biomarkers of neurological disease: an analytical perspective. Biomark Med. 2015;9:691–701.
Ornitz EM, Ritvo ER. Perceptual inconstancy in early infantile autism. The syndrome of early infant autism and its variants including certain cases of childhood schizophrenia. Arch Gen Psychiatry. 1968;18:76–98.
Little J-A. Vision in children with autism spectrum disorder: a critical review. Clin Exper Optometry. 2018;101:504–13.
Bakroon A, Lakshminarayanan V. Visual function in autism spectrum disorders: a critical review. Clin Exp Optom. 2016;99:297–308.
Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. Vision in autism spectrum disorders. Vision Res. 2009;49:2705–39.
Van der Hallen R, Manning C, Evers K, Wagemans J. Global motion perception in autism spectrum disorder: a meta-analysis. J Autism Dev Disord. 2019;49:4901–18.
Johnson BP, Lum JA, Rinehart NJ, Fielding J. Ocular motor disturbances in autism spectrum disorders: systematic review and comprehensive meta-analysis. Neurosci Biobehav Rev. 2016;69:260–79.
Chang MY, Doppee D, Yu F, Perez C, Coleman AL, Pineles SL. Prevalence of ophthalmologic diagnoses in children with autism spectrum disorder using the optum dataset: apopulation-based study. Am J Ophthalmol. 2021;221:147–53.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Kamp-Becker I, Albertowski K, Becker J, Ghahreman M, Langmann A, Mingebach T, et al. Diagnostic accuracy of the ADOS and ADOS-2 in clinical practice. Eur Child Adolesc Psychiatry. 2018;27:1193–207.
Lortie CJ. Doing meta-analysis with R - a hands-on guide. J Stat Softw Book Rev. 2022;102:1–4.
Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open. 2016;6:e011458.
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36:1–48.
Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, et al. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98.
Cochran WG. Some methods for strengthening the common χ2 Tests. Biometrics. 1954;10:417–51.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Publication bias. In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Introduction to meta-analysis. Chichester, U.K: John Wiley & Sons; 2009. pp 277–92.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63.
MA AL, Alsaqr AM. A comparative study of corneal topography in children with autism spectrum disorder: a cross-sectional study. Vision. 2021;15:5.
Anketell PM, Saunders KJ, Gallagher S, Bailey C, Little JA. Profile of refractive errors in European Caucasian children with Autistic Spectrum Disorder; increased prevalence and magnitude of astigmatism. Ophthalmic Physiol Opt. 2016;36:395–403.
Wang J, Ding G, Li Y, Hua N, Wei N, Qi X, et al. Refractive status and amblyopia risk factors in Chinese children with autism spectrum disorder. J Autism Dev Disorders. 2018;48:1530–6.
Little JA, Anketell P, Gallagher S, Saunders K. Refractive and corneal astigmatism in autistic spectrum disorder. Investig Ophthalmol Vis Sci. 2013;54:3.
Milne E, Griffiths H, Buckley D, Scope A. Vision in children and adolescents with autistic spectrum disorder: evidence for reduced convergence. J Autism Dev Disord. 2009;39:965–75.
Classen S, Monahan M, Brown KE, Hernandez S. Driving indicators in teens with attention deficit hyperactivity and/or autism spectrum disorder. Can J Occup Ther. 2013;80:274–83.
Little JA, Anketell P, Doyle L, Saunders KJ. Exploring accommodative accuracy in autism spectrum disorder. Investig Ophthalmol Vis Sci. 2014;55:3769.
Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J, et al. Prevalence of sensory impairments, physical and intellectual disabilities, and mental health in children and young people with self/proxy-reported autism: observational study of a whole country population. Autism. 2019;23:1201–9.
Swanson MW, Lee SD, Frazier MG, Bade A, Coulter RA. Vision screening among children with autism spectrum disorder. Optom Vis Sci. 2020;97:917–28.
Rydzewska E, Hughes-McCormack LA, Gillberg C, Henderson A, MacIntyre C, Rintoul J, et al. Prevalence of long-term health conditions in adults with autism: observational study of a whole country population. BMJ Open. 2018;8:e023945.
Mouridsen SE, Rich B, Isager T. Eye disorders among adult people diagnosed with infantile autism in childhood: a longitudinal case control study. Ophthalmic Epidemiol. 2017;24:332–5.
Anketell PM, Saunders KJ, Gallagher SM, Bailey C, Little JA. Accommodative function in individuals with autism spectrum disorder. Optom Vis Sci. 2018;95:193–201.
Davis RAO, Bockbrader MA, Murphy RR, Hetrick WP, O’Donnell BF. Subjective perceptual distortions and visual dysfunction in children with autism. J Autism Dev Disord. 2006;36:199–210.
Albrecht MA, Stuart GW, Falkmer M, Ordqvist A, Leung D, Foster JK, et al. Brief report: visual acuity in children with autism spectrum disorders. J Autism Dev Disord. 2014;44:2369–74.
Anketell PM, Saunders KJ, Gallagher SM, Bailey C, Little J-A. Brief report: vision in children with autism spectrum disorder: what should clinicians expect?. J Autism Dev Disord. 2015;45:3041–7.
Ashwin E, Ashwin C, Rhydderch D, Howells J, Baron-Cohen S. Eagle-eyed visual acuity: an experimental investigation of enhanced perception in autism. Biol Psychiatry. 2009;65:17–21.
Bölte S, Schlitt S, Gapp V, Hainz D, Schirman S, Poustka F, et al. A close eye on the eagle-eyed visual acuity hypothesis of autism. J Autism Dev Disord. 2012;42:726–33.
Brosnan MJ, Gwilliam LR, Walker I. Brief report: the relationship between visual acuity, the embedded figures test and systemizing in autism spectrum disorders. J Autism Dev Disord. 2012;42:2491–7.
Falkmer M, Stuart GW, Danielsson H, Bram S, Lönebrink M, Falkmer T. Visual acuity in adults with Asperger’s syndrome: no evidence for “eagle-eyed” vision. Biol Psychiatry. 2011;70:812–6.
Kéïta L, Mottron L, Bertone A. Far visual acuity is unremarkable in autism: do we need to focus on crowding? Autism Res. 2010;3:333–41.
Tavassoli T, Latham K, Bach M, Dakin SC, Baron-Cohen S. Psychophysical measures of visual acuity in autism spectrum conditions. Vision Res. 2011;51:1778–80.
Tebartz van Elst L, Bach M, Blessing J, Riedel A, Bubl E. Normal visual acuity and electrophysiological contrast gain in adults with high-functioning autism spectrum disorder. Front Hum Neurosci. 2015;9:460.
Coulter RA, Bade A, Jenewein EC, Tea YC, Mitchell GL. Near-point findings in children with autism spectrum disorder and in typical peers. Optom Vis Sci. 2021;98:384–93.
Smith D, Ropar D, Allen HA. The integration of occlusion and disparity information for judging depth in autism spectrum disorder. J Autism Dev Disord. 2017;47:3112–24.
Milne E, Scope A, Griffiths H, Codina C, Buckley D. Brief report: preliminary evidence of reduced sensitivity in the peripheral visual field of adolescents with autistic spectrum disorder. J Autism Dev Disord. 2013;43:1976–82.
Song Y, Hakoda Y, Sanefuji W, Cheng C. Can they see it? The functional field of view is narrower in individuals with autism spectrum disorder. PLoS One. 2015;10:e0133237.
García-Medina JJ, García-Piñero M, Del-Río-Vellosillo M, Fares-Valdivia J, Ragel-Hernández AB, Martínez-Saura S, et al. Comparison of foveal, macular, and peripapillary intraretinal thicknesses between autism spectrum disorder and neurotypical subjects. Invest Ophthalmol Vis Sci. 2017;58:5819–26.
Little JA, Anketell PM, Doyle L, Saunders K. Investigation of retinal thickness using OCT in Autism spectrum disorder. Investig Ophthalmol Vis Sci. 2016;57:4217.
Emberti Gialloreti L, Pardini M, Benassi F, Marciano S, Amore M, Mutolo MG, et al. Reduction in retinal nerve fiber layer thickness in young adults with autism spectrum disorders. J Autism Dev Disord. 2014;44:873–82.
Constable PA, Gaigg SB, Bowler DM, Jagle H, Thompson DA. Full-field electroretinogram in autism spectrum disorder. Documenta Ophthalmol. 2016;132:83–99.
Constable PA, Lee IO, Marmolejo-Ramos F, Skuse DH, Thompson DA. The photopic negative response in autism spectrum disorder. Clin Exp Optom. 2021;104:841–7.
Constable PA, Ritvo ER, Ritvo AR, Lee IO, McNair ML, Stahl D, et al. Light-adapted electroretinogram differences in autism spectrum disorder. J Autism Dev Disord. 2020;50:2874–85.
Garcia-Medina JJ, Rubio-Velazquez E, Lopez-Bernal MD, Parraga-Muñoz D, Perez-Martinez A, Pinazo-Duran MD, et al. Optical coherence tomography angiography of macula and optic nerve in autism spectrum disorder: a pilot study. J Clin Med. 2020;9:3123.
Franklin A, Sowden P, Burley R, Notman L, Alder E. Color perception in children with autism. J Autism Dev Disord. 2008;38:1837–47.
Franklin A, Sowden P, Notman L, Gonzalez-Dixon M, West D, Alexander I, et al. Reduced chromatic discrimination in children with autism spectrum disorders. Dev Sci. 2010;13:188–200.
Heaton P, Ludlow A, Roberson D. When less is more: poor discrimination but good colour memory in autism. Res Autism Spectrum Disord. 2008;2:147–56.
Maule J, Stanworth K, Pellicano E, Franklin A. Ensemble perception of color in autistic adults. Autism Res. 2017;10:839–51.
Zachi EC, Costa TL, Barboni MTS, Costa MF, Bonci DMO, Ventura DF. Color vision losses in autism spectrum disorders. Front Psychol. 2017;8:1127.
Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–41.
De Jonge MV, De Haan EH, Kemner C, Coppens JE, Van Den Berg TJTP, Van Engeland H. Visual information processing in high-functioning individuals with autism spectrum disorders and their parents. Neuropsychology. 2007;21:65–73.
Greenaway R, Davis G, Plaisted-Grant K. Marked selective impairment in autism on an index of magnocellular function. Neuropsychologia. 2013;51:592–600.
Guy J, Mottron L, Berthiaume C, Bertone A. The developmental trajectory of contrast sensitivity in autism spectrum disorder. Autism Res. 2016;9:866–78.
Keita L, Guy J, Berthiaume C, Mottron L, Bertone A. An early origin for detailed perception in Autism Spectrum Disorder: biased sensitivity for high-spatial frequency information. Sci Rep. 2014;4:5475.
Koh HC, Milne E, Dobkins K. Spatial contrast sensitivity in adolescents with autism spectrum disorders. J Autism Dev Disord. 2010;40:978–87.
Norton DJ, McBain RK, Murray GE, Khang J, Zong Z, Bollacke HR, et al. Normal face detection over a range of luminance contrasts in adolescents with autism spectrum disorder. Front Psychol. 2021;12:667359.
Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–53.
Robertson CE, Kravitz DJ, Freyberg J, Baron-Cohen S, Baker CI. Tunnel vision: sharper gradient of spatial attention in autism. J Neurosci. 2013;33:6776–81.
Davis RA, Bockbrader MA, Murphy RR, Hetrick WP, O’Donnell BF. Subjective perceptual distortions and visual dysfunction in children with autism. J Autism Dev Disord. 2006;36:199–210.
Koh HC, Milne E, Dobkins K. Contrast sensitivity for motion detection and direction discrimination in adolescents with autism spectrum disorders and their siblings. Neuropsychologia. 2010;48:4046–56.
Searle A, Rowe FJ. Vergence neural pathways: a systematic narrative literature review. Neuroophthalmology. 2016;40:209–18.
Congdon NG, Patel N, Esteso P, Chikwembani F, Webber F, Msithini RB, et al. The association between refractive cutoffs for spectacle provision and visual improvement among school-aged children in South Africa. Br J Ophthalmol. 2008;92:13–8.
McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol. 2015;5:439–73.
Arora I, Bellato A, Ropar D, Hollis C, Groom MJ. Is autonomic function during resting-state atypical in Autism: a systematic review of evidence. Neurosci Biobehav Rev. 2021;125:417–41.
Cheng YC, Huang YC, Huang WL. Heart rate variability in individuals with autism spectrum disorders: a meta-analysis. Neurosci Biobehav Rev. 2020;118:463–71.
de Vries L, Fouquaet I, Boets B, Naulaers G, Steyaert J. Autism spectrum disorder and pupillometry: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;120:479–508.
Ronconi L, Gori S, Ruffino M, Molteni M, Facoetti A. Zoom-out attentional impairment in children with autism spectrum disorder. Cortex. 2013;49:1025–33.
Cameron JR, Tatham AJ. A window to beyond the orbit: the value of optical coherence tomography in non-ocular disease. Acta Ophthalmol. 2016;94:533–9.
Bubl E, Dörr M, Philipsen A, Ebert D, Bach M, van Elst LT. Retinal contrast transfer functions in adults with and without ADHD. PLoS One. 2013;8:e61728.
Bubl E, Dörr M, Riedel A, Ebert D, Philipsen A, Bach M, et al. Elevated background noise in adult attention deficit hyperactivity disorder is associated with inattention. PLoS One. 2015;10:e0118271.
Werner AL, Tebartz van Elst L, Ebert D, Friedel E, Bubl A, Clement HW, et al. Normalization of increased retinal background noise after ADHD treatment: a neuronal correlate. Schizophr Res. 2020;219:77–83.
Happé F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36:5–25.
Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006;36:27–43.
Samson F, Mottron L, Soulières I, Zeffiro TA. Enhanced visual functioning in autism: an ALE meta-analysis. Hum Brain Mapp. 2012;33:1553–81.
Jassim N, Baron-Cohen S, Suckling J. Meta-analytic evidence of differential prefrontal and early sensory cortex activity during non-social sensory perception in autism. Neurosci Biobehav Rev. 2021;127:146–57.
Todorova GK, Hatton REM, Pollick FE. Biological motion perception in autism spectrum disorder: a meta-analysis. Mol Autism. 2019;10:49.
Riddiford JA, Enticott PG, Lavale A, Gurvich C. Gaze and social functioning associations in autism spectrum disorder: A systematic review and meta-analysis. Autism Res. 2022;15:1380–446.
Setien-Ramos I, Lugo-Marín J, Gisbert-Gustemps L, Díez-Villoria E, Magán-Maganto M, Canal-Bedia R, et al. Eye-tracking studies in adults with autism spectrum disorder: a systematic review and meta-analysis. J Autism Dev Disord. 2022;53:2430–43.
Thye MD, Bednarz HM, Herringshaw AJ, Sartin EB, Kana RK. The impact of atypical sensory processing on social impairments in autism spectrum disorder. Dev Cogn Neurosci. 2018;29:151–67.
Ronconi L, Molteni M, Casartelli L. Building blocks of others’ understanding: a perspective shift in investigating social-communicative deficit in autism. Front Hum Neurosci. 2016;10:144.
Jin X, Simmons SK, Guo A, Shetty AS, Ko M, Nguyen L, et al. In vivo Perturb-Seq, reveals neuronal and glial abnormalities associated with autism risk genes. Science. 2020;370:eaaz6063.
Scotland P, Zhou D, Benveniste H, Bennett V. Nervous system defects of AnkyrinB (-/-) mice suggest functional overlap between the cell adhesion molecule L1 and 440-kD AnkyrinB in premyelinated axons. J Cell Biol. 1998;143:1305–15.
Elsabbagh M, Fernandes J, Webb SJ, Dawson G, Charman T, Johnson MH. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol Psychiatry. 2013;74:189–94.
Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504:427–31.
Chokron S, Kovarski K, Zalla T, Dutton GN. The inter-relationships between cerebral visual impairment, autism and intellectual disability. Neurosci Biobehav Rev. 2020;114:201–10.
McGaha CG, Farran DC. Interactions in an inclusive classroom: the effects of visual status and setting. J Vis Impair Blindness. 2001;95:80–94.
Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, Kendler KS. Psychiatric genetics and the structure of psychopathology. Mol Psychiatry. 2019;24:409–20.
Bellato A, Perna J, Ganapathy PS, Solmi M, Zampieri A, Cortese S, et al. Association between ADHD and vision problems. A systematic review and meta-analysis. Mol Psychiatry. 2023;28:410–22.
Császár N, Kapócs G, Bókkon I. A possible key role of vision in the development of schizophrenia. Rev Neurosci. 2019;30:359–79.
van Splunder J, Stilma JS, Bernsen RM, Evenhuis HM. Prevalence of visual impairment in adults with intellectual disabilities in the Netherlands: cross-sectional study. Eye. 2006;20:1004–10.
Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6:819–29.
Johnson MH, Gliga T, Jones E, Charman T. Annual research review: infant development, autism, and ADHD-early pathways to emerging disorders. J Child Psychol Psychiatry. 2015;56:228–47.
Visser JC, Rommelse NN, Greven CU, Buitelaar JK. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: a review of unique and shared characteristics and developmental antecedents. Neurosci Biobehav Rev. 2016;65:229–63.
Karmiloff-Smith A. From constructivism to neuroconstructivism: the activity-dependent structuring of the human brain. After Piaget. Piscataway, NJ, US: Transaction Publishers; 2012. p. 1–14.
Simms MD. When autistic behavior suggests a disease other than classic autism. Pediatr Clin North Am. 2017;64:127–38.
Landa RJ. Efficacy of early interventions for infants and young children with, and at risk for, autism spectrum disorders. Int Rev Psychiatry. 2018;30:25–39.
O’Connor AR, Stephenson T, Johnson A, Tobin MJ, Moseley MJ, Ratib S, et al. Long-term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics. 2002;109:12–8.
Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128:344–55.
Maeyama K, Tomioka K, Nagase H, Yoshioka M, Takagi Y, Kato T, et al. Congenital cytomegalovirus infection in children with autism spectrum disorder: systematic review and meta-analysis. J Autism Dev Disord. 2018;48:1483–91.
Mawson AR, Croft AM. Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int J Environ Res Public Health. 2019;16:3543.
Mohiuddin S, Ghaziuddin M. Psychopharmacology of autism spectrum disorders: a selective review. Autism. 2013;17:645–54.
Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40.
Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24:501–26.
Bahali K, Ipek H, Yalcin O, Orum O. Atomoxetine-induced mydriasis in a child patient. Eur Child Adolesc Psychiatry. 2014;23:1231–2.
Lu CK, Kuang TM, Chou JC. Methylphenidate (Ritalin)-associated cataract and glaucoma. J Chin Med Assoc. 2006;69:589–90.
Soyer J, Jean-Louis J, Ospina LH, Bélanger SA, Bussières JF, Kleiber N. Visual disorders with psychostimulants: a paediatric case report. Paediatr Child Health. 2019;24:153–5.
Westreich D. Berkson’s bias, selection bias, and missing data. Epidemiology. 2012;23:159–64.
Acknowledgements
No funding has been received for the present study. We thank Matthew A. Albrecht, Paul A. Constable, Luc Kéïta, Julie-Anne Little and their colleagues, for providing additional details about their studies and helping us to determine the eligibility for the systematic review and meta-analyses, and for providing additional data when these were not present in the original papers.
Author information
Authors and Affiliations
Contributions
JP: Investigation, Data Curation, Visualization, Writing - Original Draft, Writing - Review & Editing. AB: Formal analysis, Investigation, Data Curation, Visualization, Writing - Original Draft, Writing - Review & Editing. PSG: Validation, Writing - Review & Editing. MS: Validation, Writing - Review & Editing. AZ: Validation, Writing - Review & Editing. SVF: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Supervision, Project administration. SC: Conceptualization, Methodology, Writing - Original Draft, Writing - Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Competing interests
In the past year, SVF received income, potential income, travel expenses continuing education support and/or research support from Aardvark, Aardwolf, Akili, Atentiv, Corium, Genomind, Ironshore, Medice, Noven, Otsuka, Sandoz, Sky Therapeutics, Supernus, Tris, and Vallon. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received support from: Alcobra, Arbor, Aveksham, Axsome, CogCubed, Eli Lilly, Enzymotec, Impact, Janssen, KemPharm, Lundbeck/Takeda, Shire/Takeda, McNeil, NeuroLifeSciences, Neurovance, Novartis, Pfizer, Rhodes, Shire, and Sunovion. He also receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health; Oxford University Press: Schizophrenia: The Facts; and Elsevier: ADHD: Non-Pharmacologic Interventions. In addition, he is the program director of www.adhdinadults.com. SVF is supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 965381; NIMH grants U01AR076092-01A1, 1R21MH1264940, R01MH116037; 1R01NS128535 – 01; Oregon Health and Science University, Otsuka Pharmaceuticals, Noven Pharmaceuticals Incorporated, and Supernus Pharmaceutical Company. SC declares honoraria and reimbursement for travel and accommodation expenses for lectures from the following non-profit associations: Association for Child and Adolescent Central Health (ACAMH), Canadian ADHD Alliance Resource (CADDRA), and British Association of Pharmacology (BAP), for educational activity on ADHD. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the Discussion paragraph of the abstract, the sentence ‘Further research should clarify the causal relationship, if any, between ASD and problems of vision and if problems of vision during early life’. should have read ‘Further research should clarify the causal relationship, if any, between ASD and problems of vision during early life’.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perna, J., Bellato, A., Ganapathy, P.S. et al. Association between Autism Spectrum Disorder (ASD) and vision problems. A systematic review and meta-analysis. Mol Psychiatry 28, 5011–5023 (2023). https://doi.org/10.1038/s41380-023-02143-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02143-7