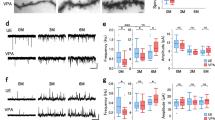
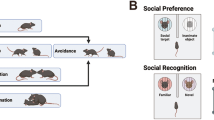
Abstract
Autism spectrum disorder (ASD) is a prevalent and poorly understood neurodevelopmental disorder. There are currently no laboratory-based diagnostic tests to detect ASD, nor are there any disease-modifying medications that effectively treat ASD’s core behavioral symptoms. Scientific progress has been impeded, in part, by overreliance on model organisms that fundamentally lack the sophisticated social and cognitive abilities essential for modeling ASD. We therefore saw significant value in studying naturally low-social rhesus monkeys to model human social impairment, taking advantage of a large outdoor-housed colony for behavioral screening and biomarker identification. Careful development and validation of our animal model, combined with a strong commitment to evaluating the translational utility of our preclinical findings directly in patients with ASD, yielded a robust neurochemical marker (cerebrospinal fluid vasopressin concentration) of trans-primate social impairment and a first-in-class medication (intranasal vasopressin) shown in a small phase 2a pilot trial to improve social abilities in children with ASD. This translational research approach stands to advance our understanding of ASD in a manner not readily achievable with existing animal models, and can be adapted to investigate a variety of other human brain disorders which currently lack valid preclinical options, thereby streamlining translation and amplifying clinical impact more broadly.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
APA. Diagnostic and Statistical Manual of Mental Disorders. Fifth edn. American Psychiatric Association: Washington, DC, 2013.
Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2018. MMWR Surveill Summ. 2021;70:1–16.
Leigh JP, Du J. Brief report: forecasting the economic burden of Autism in 2015 and 2025 in the United States. J Autism Dev Disord. 2015;45:4135–9.
Gaugler T, Klei L, Sanders SJ, Bodea CA, Goldberg AP, Lee AB, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–5.
Robinson EB, St Pourcain B, Anttila V, Kosmicki JA, Bulik-Sullivan B, Grove J, et al. Genetic risk for autism spectrum disorders and neuropsychiatric variation in the general population. Nat Genet. 2016;48:552–5.
Iakoucheva LM, Muotri AR, Sebat J. Getting to the Cores of Autism. Cell. 2019;178:1287–98.
Rogers SJ, Vismara L Interventions for Infants and Toddlers at Risk for Autism Spectrum Disorder. In: Society AC (ed). Handbook of Autism and Pervasive Developmental Disorders, Fourth Edition 2014.
Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. 2012;10:CD009260.
Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–21.
Aldinger KA, Qiu S. New mouse genetic model duplicates human 15q11-13 autistic phenotypes, or does it? Dis Model Mech. 2010;3:3–4.
Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, et al. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci USA. 2011;108:17076–81.
Capitanio JP, Emborg ME. Contributions of non-human primates to neuroscience research. Lancet. 2008;371:1126–35.
Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Disco. 2004;3:711–5.
Garner JP. The significance of meaning: why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? ILAR J / Natl Res Counc, Inst Lab Anim Resour. 2014;55:438–56.
Zahs KR, Ashe KH. ‘Too much good news’ - are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer’s disease? Trends Neurosci. 2010;33:381–9.
Garner JP, Gaskill BN, Weber EM, Ahloy-Dallaire J, Pritchett-Corning KR. Introducing Therioepistemology: the study of how knowledge is gained from animal research. Lab Anim (NY). 2017;46:103–13.
McKinney WT Jr., WE Bunney Jr. Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 1969;21:240–8.
Willner P. The validity of animal models of depression. Psychopharmacol (Berl). 1984;83:1–16.
Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30:97–117.
Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2:295–302.
Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br Med Bull. 1997;53:170–84.
Rutter M, Andersen-Wood L, Beckett C, Bredenkamp D, Castle J, Groothues C, et al. Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. J Child Psychol Psychiatry. 1999;40:537–49.
Capitanio JP. Social processes and disease in nonhuman primates: introduction to the special section. Am J Primatol. 2012;74:491–6.
Bettelheim B. The empty fortress: infantile autism and the birth of the self. Free Press: New York, NY, 1967.
DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, et al. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–906.
Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J, et al. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet. 1999;65:493–507.
Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, et al. Variable expression of the autism broader phenotype: findings from extended pedigrees. J Child Psychol Psychiatry. 2000;41:491–502.
Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: evidence from a family history study of multiple-incidence autism families. Am J Psychiatry. 1997;154:185–90.
Bishop DV, Maybery M, Maley A, Wong D, Hill W, Hallmayer J. Using self-report to identify the broad phenotype in parents of children with autistic spectrum disorders: a study using the Autism-Spectrum Quotient. J Child Psychol Psychiatry. 2004;45:1431–6.
Constantino JN, Lajonchere C, Lutz M, Gray T, Abbacchi A, McKenna K, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163:294–6.
Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–30.
Constantino JN, Todd RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. 2005;57:655–60.
Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17.
Robinson EB, Koenen KC, McCormick MC, Munir K, Hallett V, Happe F, et al. Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%). Arch Gen Psychiatry. 2011;68:1113–21.
Lundstrom S, Chang Z, Rastam M, Gillberg C, Larsson H, Anckarsater H, et al. Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry. 2012;69:46–52.
Müller E, Schuler A, Yates GB. Social challenges and supports from the perspective of individuals with Asperger syndrome and other Autism spectrum disabilities. Autism. 2008;12:173–90.
Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, T Hart BA, et al. Why primate models matter. Am J Primatol. 2014;76:801–27.
Tigges J, Gordon TP, McClure HM, Hall EC, Paters AA. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15:263–73.
Hull L, Petrides KV, Mandy W. The Female Autism Phenotype and Camouflaging: a Narrative Review. Rev J Autism Developmental Disord. 2020;7:306–17.
Capitanio JP. Sociability and responses to video playbacks in adult male rhesus monkeys (Macaca mulatta). Primates. 2002;43:169–77.
Sclafani V, Del Rosso LA, Seil SK, Calonder LA, Madrid JE, Bone KJ, et al. Early Predictors of Impaired Social Functioning in Male Rhesus Macaques (Macaca mulatta). PLoS One. 2016;11:e0165401.
Parker KJ, Garner JP, Oztan O, Tarara ER, Li J, Sclafani V, et al. Arginine vasopressin in cerebrospinal fluid is a marker of sociality in nonhuman primates. Sci Transl Med. 2018;10:eaam9100.
Myers AK, Talbot CF, Del Rosso LA, Maness AC, Simmons SMV, Garner JP, et al. Assessment of medical morbidities in a rhesus monkey model of naturally occurring low sociality. Autism Res. 2021;14:1332–46.
Jain A, Spencer D, Yang W, Kelly JP, Newschaffer CJ, Johnson J, et al. Injuries among children with autism spectrum disorder. Acad Pediatr. 2014;14:390–7.
Cavalari RN, Romanczyk RG. Caregiver perspectives on unintentional injury risk in children with an autism spectrum disorder. J Pediatr Nurs. 2012;27:632–41.
Lee LC, Harrington RA, Chang JJ, Connors SL. Increased risk of injury in children with developmental disabilities. Res Dev Disabil. 2008;29:247–55.
McDermott S, Zhou L, Mann J. Injury treatment among children with autism or pervasive developmental disorder. J Autism Dev Disord. 2008;38:626–33.
Feczko EJ, Bliss-Moreau E, Walum H, Pruett JR Jr., Parr LA. The Macaque Social Responsiveness Scale (mSRS): A Rapid Screening Tool for Assessing Variability in the Social Responsiveness of Rhesus Monkeys (Macaca mulatta). PLoS One. 2016;11:e0145956.
Talbot CF, Garner JP, Maness AC, McCowan B, Capitanio JP, Parker KJ. A psychometrically robust screening tool to rapidly identify socially impaired monkeys in the general population. Autism Res. 2020;13:1465–75.
Constantino JN, Gruber CP. Social Responsiveness Scale, Second Edition. Western Psychological Services 2012.
Constantino JN, Abbacchi AM, Lavesser PD, Reed H, Givens L, Chiang L, et al. Developmental course of autistic social impairment in males. Dev Psychopathol. 2009;21:127–38.
Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33:427–33.
Connolly JJ, Glessner JT, Hakonarson H. A genome-wide association study of autism incorporating autism diagnostic interview-revised, autism diagnostic observation schedule, and social responsiveness scale. Child Dev. 2013;84:17–33.
Constantino JN. The quantitative nature of autistic social impairment. Pediatr Res. 2011;69:55R–62R.
Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, Santangelo SL. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2014;71:936–42.
Garner JP, Talbot CF, Del Rosso LA, McCowan B, Haig D, Capitanio JP, et al. Rhesus macaque social functioning is paternally, but not maternally, inherited by sons: Potential implications for autism. under review.
Capitanio JP. Variation in BioBehavioral Organization. In: Schapiro S. (ed). Handbook of Primate Behavioral Management. CRC Press: Boca Raton, FL, 2017, pp 55-73.
Jones W, Klin A. Attention to eyes is present but in decline in 2-6-month-old infants later diagnosed with autism. Nature. 2013;504:427–31.
Keehn B, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder. Autism Res. 2015;8:187–98.
Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014;1580:199–218.
Baribeau DA, Anagnostou E. Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits. Front Neurosci. 2015;9:335.
Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–7.
Subramanian M, Timmerman CK, Schwartz JL, Pham DL, Meffert MK. Characterizing autism spectrum disorders by key biochemical pathways. Front Neurosci. 2015;9:313.
Oztan O, Talbot CF, Argilli E, Maness AC, Simmons SM, Mohsin N, et al. Autism-associated biomarkers: test-retest reliability and relationship to quantitative social trait variation in rhesus monkeys. Mol Autism. 2021;12:50.
Oztan O, Garner JP, Partap S, Sherr EH, Hardan AY, Farmer C, et al. Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann Neurol. 2018;84:611–5.
Shoffner J, Trommer B, Thurm A, Farmer C, Langley WA, 3rd, Soskey L et al. CSF concentrations of 5-methyltetrahydrofolate in a cohort of young children with autism. Neurology. 2016;86:2258–63.
Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Hormones Behav. 2012;61:283–92.
Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–8.
Parker KJ, Lee TM. Central vasopressin administration regulates the onset of facultative paternal behavior in microtus pennsylvanicus (meadow voles). Horm Behav. 2001;39:285–94.
Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol. 2004;29:483–93.
Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, et al. Atypical social development in vasopressin-deficient brattleboro rats. eNeuro. 2016;3:ENEURO.0150-15.2016.
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4.
Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701.
Oztan O, Garner JP, Constantino JN, Parker KJ. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc Natl Acad Sci USA. 2020;117:10609–13.
Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, et al. Arginine vasopressin is a blood-based biomarker of social functioning in children with Autism. PLoS One. 2015;10:e0132224.
Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–73.
Martin J, Kagerbauer SM, Schuster T, Blobner M, Kochs EF, Landgraf R. Vasopressin and oxytocin in CSF and plasma of patients with aneurysmal subarachnoid haemorrhage. Neuropeptides. 2014;48:91–96.
Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol. 2003;29:563–73.
Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharm. 2012;3:46–46.
Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview - Structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13.
Carson DS, Howerton CL, Garner JP, Hyde SA, Clark CL, Hardan AY, et al. Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides. 2014;61:12–16.
Bartrons J, Figueras J, Jimenez R, Gaya J, Cruz M. Vasopressin in cerebrospinal fluid of newborns with hypoxic-ischemic encephalopathy. Preliminary report. J Perinat Med. 1993;21:399–403.
Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Res. 1986;375:363–7.
Caffe AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–25.
Rogers CN, Ross AP, Sahu SP, Siegel ER, Dooyema JM, Cree MA, et al. Oxytocin- and arginine vasopressin-containing fibers in the cortex of humans, chimpanzees, and rhesus macaques. Am J Primatol. 2018;80:e22875.
Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–8.
Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124:159–63.
Freeman SM, Inoue K, Smith AL, Goodman MM, Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology. 2014;45:128–41.
Rogers Flattery CN, Coppeto DJ, Inoue K, Rilling JK, Preuss TM, Young LJ Distribution of brain oxytocin and vasopressin V1a receptors in chimpanzees (Pan troglodytes): comparison with humans and other primate species. Brain Struct Funct. 2021. https://doi.org/10.1007/s00429-021-02369-7. Online ahead of print.
Guastella AJ, Kenyon AR, Alvares GA, Carson DS, Hickie IB. Intranasal arginine vasopressin enhances the encoding of happy and angry faces in humans. Biol Psychiatry. 2010;67:1220–2.
Guastella AJ, Kenyon AR, Unkelbach C, Alvares GA, Hickie IB. Arginine Vasopressin selectively enhances recognition of sexual cues in male humans. Psychoneuroendocrinology. 2011;36:294–7.
Brunnlieb C, Nave G, Camerer CF, Schosser S, Vogt B, Munte TF, et al. Vasopressin increases human risky cooperative behavior. Proc Natl Acad Sci USA. 2016;113:2051–6.
Tsikunov SG, Belokoskova SG. Psychophysiological analysis of the influence of vasopressin on speech in patients with post-stroke aphasias. Span J Psychol. 2007;10:178–88.
Laczi F, Valkusz Z, Laszlo FA, Wagner A, Jardanhazy T, Szasz A, et al. Effects of lysine-vasopressin and 1-deamino-8-D-arginine-vasopressin on memory in healthy individuals and diabetes insipidus patients. Psychoneuroendocrinology. 1982;7:185–93.
Quintana DS, Guastella AJ, Westlye LT, Andreassen OA. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol Psychiatry. 2016;21:29–38.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–6.
Parker KJ, Oztan O, Libove RA, Mohsin N, Karhson DS, Sumiyoshi RD, et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11:eaau7356.
Talbot CF, Maness AC, Capitanio JP, Parker KJ. The factor structure of the macaque social responsiveness scale-revised predicts social behavior and personality dimensions. Am J Primatol. 2021;83:e23234.
Loomes R, Hull L, Mandy WPL. What is the male-to-female ratio in autism spectrum disorder? a systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:466–74.
Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99.
Acknowledgements
I am grateful to the research collaborators who thought with me and made this body of work possible. They include: Joe Garner, Ozge Oztan, John Capitanio, Antonio Hardan, Elliott Sherr, Kate Talbot, Jennifer Phillips, Sonia Partap, Valentina Sclafani, Sue Swedo, John Constantino, Annie Penn, Dean Carson, Dan Bowling, Laura Del Rosso, Robin Libove, and Adam Myers. I also thank my departmental colleagues Carl Feinstein, David Hong, and Lawrence Fung for their thoughtful discussions on both ASD and the benefits and limitations of animal models. This work has been supported by the National Institutes of Health (R01HD091972; R01HD087048; R21HD083629; R21MH100387; R21HD079095; P51OD011107; R24OD010962), the Simons Foundation (SFARI#s 627146; 342873; 274472; 93231), the Department of Défense (W81XWH-21-1-0210), several family foundations (Weston Havens Foundation; Mosbacher Family Fund for Autism Research; The Yani Calmidis Memorial Fund for Autism Research; The Gupta Foundation; The Peter and Stacy Sullivan Foundation), and Stanford University (Department of Psychiatry; Maternal Child Health Research Institute; Bio-X NeuroVentures Program; Lucile Packard Children’s Hospital Pediatric Neurosciences Fund; The Katherine D. McCormick Fund; Wu Tsai Neurosciences Institute).
Author information
Authors and Affiliations
Contributions
KJP conceptualized, investigated, wrote, reviewed, and edited this manuscript.
Corresponding author
Ethics declarations
Competing interests
The Board of Trustees of the Leland Stanford Junior University has filed patent applications related to data reviewed herein: PCT/US2019/019029 (“Methods for diagnosing and determining severity of an autism spectrum disorder”) and PCT/US2019/041250 (“Intranasal Vasopressin Treatment for Social Deficits in Children with Autism”). These patents have not been granted, nor licensed, and the author is not receiving any financial compensation at this time.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parker, K.J. Leveraging a translational research approach to drive diagnostic and treatment advances for autism. Mol Psychiatry 27, 2650–2658 (2022). https://doi.org/10.1038/s41380-022-01532-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01532-8
This article is cited by
-
Naturally occurring low sociality in female rhesus monkeys: A tractable model for autism or not?
Molecular Autism (2024)