Abstract
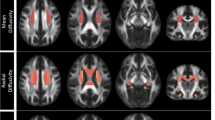
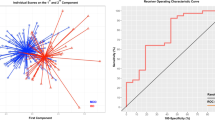
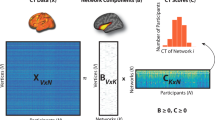
Abnormalities in brain structural measures, such as cortical thickness and subcortical volumes, are observed in patients with major depressive disorder (MDD) who also often show heterogeneous clinical features. This study seeks to identify the multivariate associations between structural phenotypes and specific clinical symptoms, a novel area of investigation. T1-weighted magnetic resonance imaging measures were obtained using 3 T scanners for 178 unmedicated depressed patients at four academic medical centres. Cortical thickness and subcortical volumes were determined for the depressed patients and patients’ clinical presentation was characterized by 213 item-level clinical measures, which were grouped into several large, homogeneous categories by K-means clustering. The multivariate correlations between structural and cluster-level clinical-feature measures were examined using canonical correlation analysis (CCA) and confirmed with both 5-fold and leave-one-site-out cross-validation. Four broad types of clinical measures were detected based on clustering: an anxious misery composite (composed of item-level depression, anxiety, anhedonia, neuroticism and suicidality scores); positive personality traits (extraversion, openness, agreeableness and conscientiousness); reported history of physical/emotional trauma; and a reported history of sexual abuse. Responses on the item-level anxious misery measures were negatively associated with cortical thickness/subcortical volumes in the limbic system and frontal lobe; reported childhood history of physical/emotional trauma and sexual abuse measures were negatively correlated with entorhinal thickness and left hippocampal volume, respectively. In contrast, the positive traits measures were positively associated with hippocampal and amygdala volumes and cortical thickness of the highly-connected precuneus and cingulate cortex. Our findings suggest that structural brain measures may reflect neurobiological mechanisms underlying MDD features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
APA. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition DSM-5. American Psychiatric Association. 2013.
Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30.
Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119–38.
Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. 2012;138:9–18.
Lai CH. Gray matter volume in major depressive disorder: a meta-analysis of voxel-based morphometry studies. Psychiatry Res. 2013;211:37–46.
Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607.
Abe C, Ekman CJ, Sellgren C, Petrovic P, Ingvar M, Landen M. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 2016;41:240–50.
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9.
Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 2016;21:806–12.
Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–52.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66.
Arnone D, McIntosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur Neuropsychopharmacol. 2012;22:1–16.
Hotelling H. Relations between two sets of variates*. Biometrika. 1936;28:321–77.
Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA, et al. Moderation of the relationship between reward expectancy and prediction error-related ventral striatal reactivity by anhedonia in unmedicated major depressive disorder: findings from the EMBARC study. Am J Psychiatry. 2015;172:881–91.
Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, et al. Neural correlates of three promising endophenotypes of depression: evidence from the EMBARC Study. Neuropsychopharmacol. 2016;41:454–63.
Pizzagalli DA, Webb CA, Dillon DG, Tenke CE, Kayser J, Goer F, et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: a randomized clinical trial. JAMA Psychiatry. 2018;75:547–54.
Whitton AE, Webb CA, Dillon DG, Kayser J, Rutherford A, Goer F, et al. Pretreatment rostral anterior cingulate cortex connectivity with salience network predicts depression recovery: findings From the EMBARC randomized clinical trial. Biol Psychiatry. 2019;85:872–80.
Ulke C, Tenke CE, Kayser J, Sander C, Bottger D, Wong LYX, et al. Resting EEG measures of brain arousal in a multisite study of major depression. Clin EEG Neurosci. 2019;50:3–12.
Chase HW, Fournier JC, Greenberg T, Almeida JR, Stiffler R, Zevallos CR, et al. Accounting for dynamic fluctuations across time when examining fMRI test-retest reliability: analysis of a reward paradigm in the EMBARC study. PLoS ONE. 2015;10:e0126326.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20.
Yu M, Linn KA, Cook PA, Phillips ML, McInnis M, Fava M, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum Brain Mapp. 2018;39:4213–27.
Yu M, Linn KA, Shinohara RT, Oathes DJ, Cook PA, Duprat R, et al. Childhood trauma history is linked to abnormal brain connectivity in major depression. Proc Natl Acad Sci USA. 2019;116:8582–90.
Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, et al. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp. 2016;37:2385–97.
Savalia NK, Agres PF, Chan MY, Feczko EJ, Kennedy KM, Wig GS. Motion-related artifacts in structural brain images revealed with independent estimates of in-scanner head motion. Hum Brain Mapp. 2017;38:472–92.
Weissman MM, Wickramaratne P, Merikangas KR, Leckman JF, Prusoff BA, Caruso KA, et al. Onset of major depression in early adulthood. Increase Fam Load Specif Arch Gen Psychiatry. 1984;41:1136–43.
Levinson DF, Evgrafov OV, Knowles JA, Potash JB, Weissman MM, Scheftner WA, et al. Genetics of recurrent early-onset major depression (GenRED): significant linkage on chromosome 15q25-q26 after fine mapping with single nucleotide polymorphism markers. Am J psychiatry. 2007;164:259–64.
Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–8.
Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17.
Jamieson A, Goodwill AM, Termine M, Campbell S, Szoeke C. Depression related cerebral pathology and its relationship with cognitive functioning: a systematic review. J Affect Disord. 2019;250:410–8.
Suh JS, Schneider MA, Minuzzi L, MacQueen GM, Strother SC, Kennedy SH, et al. Cortical thickness in major depressive disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:287–302.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–13.
Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–79.
Wang H, Suh JW, Das SR, Pluta JB, Craige C, Yushkevich PA. Multi-Atlas Segmentation with Joint Label Fusion. IEEE Trans Pattern Anal Mach Intell. 2013;35:611–23.
Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19:1498–507.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2017;167:104–20.
Rousseeuw PJ. Silhouettes - a graphical aid to the interpretation and validation of cluster-analysis. J Comput Appl Math. 1987;20:53–65.
Calinski T, Harabasz J. A dendrite method for cluster analysis. Commun Stat - Theory Methods. 1974;3:1–27.
Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging. Hum Brain Mapp. 2001;15:1–25.
Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–7.
Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300.
Dwyer DB, Falkai P, Koutsouleris N. Machine learning approaches for clinical psychology and psychiatry. Annu Rev Clin Psychol. 2018;14:91–118.
Hubert L, Arabie P. Comparing partitions. J Classif. 1985;2:193–218.
Ana L, Jain AK. Proceedings 2003 IEEE Computer Society Conference on Computer Vision and Pattern Recognition. 2003;2(II-128).
Xia M, Si T, Sun X, Ma Q, Liu B, Wang L, et al. Reproducibility of functional brain alterations in major depressive disorder: evidence from a multisite resting-state functional MRI study with 1,434 individuals. Neuroimage. 2019;189:700–14.
Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9:2023–8.
McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54.
Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015;77:285–94.
Sapolsky RM. Stress hormones: good and bad. Neurobiol Dis. 2000;7:540–2.
Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry. 2014;71:917–25.
McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacol. 2016;41:3–23.
Opel N, Redlich R, Dohm K, Zaremba D, Goltermann J, Repple J, et al. Mediation of the influence of childhood maltreatment on depression relapse by cortical structure: a 2-year longitudinal observational study. Lancet Psychiatry. 2019;6:318–26.
Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17:652–66.
Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35.
Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–90.
Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA. 2012;109:E563–72.
Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–5.
Papmeyer M, Giles S, Sussmann JE, Kielty S, Stewart T, Lawrie SM, et al. Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol Psychiatry. 2015;78:58–66.
Meier TB, Drevets WC, Wurfel BE, Ford BN, Morris HM, Victor TA, et al. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav Immun. 2016;53:39–48.
Won E, Choi S, Kang J, Lee MS, Ham BJ. Regional cortical thinning of the orbitofrontal cortex in medication-naive female patients with major depressive disorder is not associated with MAOA-uVNTR polymorphism. Ann Gen Psychiatry. 2016;15:26.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–82.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106:1942–7.
Nunes PM, Wenzel A, Borges KT, Porto CR, Caminha RM, de Oliveira IR. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: a meta-analysis. J Pers Disord. 2009;23:333–45.
Cai W, Leung H. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. Soc Cogn Affect Neurosci. 2006;1:229–34.
Li Q, Yang G, Qi Y, Cole M, X L. Conflict detection and resolution rely on a combination of common and distinct cognitive control networks. PLoS ONE. 2011;6:e20840.
Saxe GB, de Kirby K. Cultural context of cognitive development. Wiley Interdiscip Rev Cogn Sci. 2014;5:447–61.
Maliia MD, Donos C, Barborica A, Popa I, Ciurea J, Cinatti S, et al. Functional mapping and effective connectivity of the human operculum. Cortex. 2018;109:303–21.
Chen T, Cai W, Ryali S, Supekar K, Menon V. Distinct global brain dynamics and spatiotemporal organization of the salience network. PLoS Biol. 2016;14:e1002469.
Marquand AF, Haak KV, Beckmann CF. Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nat Hum Behav. 2017;1:0146.
Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38.
Bzdok D, Meyer-Lindenberg A. Machine learning for precision psychiatry: opportunities and challenges. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:223–30.
Davatzikos C. Machine learning in neuroimaging: progress and challenges. Neuroimage. 2019;197:652–6.
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–76.
Smith SM, Nichols TE. Statistical challenges in “Big Data” human neuroimaging. Neuron. 2018;97:263–8.
Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu J, et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–36.
Acknowledgements
We acknowledge the following support: U01 MH109991 (YIS); R01 NS085211, R01 NS060910, R01 MH112847 and RG-1707– 28586 (RTS); R01-MH111886 (DO); U01 MH092250 (MMW). TMM is supported by the Lifespan Brain Institute (LiBI) of the Children’s Hospital of Philadelphia. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. We thank Maria Prociuk for her assistance with the preparation and submission of the manuscript. We thank the EMBARC teams for collecting the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Oquendo receives royalties for the use of the Columbia Suicide Severity Rating Scale and her family owns stock in Bristol-Myers Squibb. Dr. Weissman has received funding from the Interstitial Cystitis Association, NARSAD, the National Institute on Drug Abuse, NIMH, the Sackler Foundation, and the Templeton Foundation; and she receives royalties from American Psychiatric Publishing, MultiHealth Systems, Oxford University Press, and Perseus Press., Dr. Shinohara has received consulting income from Genentech/Roche and editorial/reviewership income from the American Medical Association and Research Square. All other authors report no financial relationships with commercial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, M., Cullen, N., Linn, K.A. et al. Structural brain measures linked to clinical phenotypes in major depression replicate across clinical centres. Mol Psychiatry 26, 2764–2775 (2021). https://doi.org/10.1038/s41380-021-01039-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-021-01039-8
This article is cited by
-
Social Anhedonia: (f)MRI Studies
Neuroscience and Behavioral Physiology (2023)
-
Connectomics-based resting-state functional network alterations predict suicidality in major depressive disorder
Translational Psychiatry (2023)
-
Deriving psychiatric symptom-based biomarkers from multivariate relationships between psychophysiological and biochemical measures
Neuropsychopharmacology (2022)
-
A cross-cohort replicable and heritable latent dimension linking behaviour to multi-featured brain structure
Communications Biology (2022)
-
The human connectome in Alzheimer disease — relationship to biomarkers and genetics
Nature Reviews Neurology (2021)