Abstract
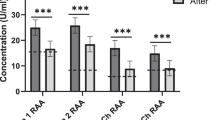
Current therapeutic approaches to Alzheimer disease (AD) remain disappointing and, hence, there is an urgent need for effective treatments. Here, we provide a perspective review on the emerging role of “metabolic inflammation” and stress as a key factor in the pathogenesis of AD and propose a novel rationale for correction of metabolic inflammation, increase resilience and potentially slow-down or halt the progression of the neurodegenerative process. Based on recent evidence and observations of an early pilot trial, we posit a potential use of extracorporeal apheresis in the prevention and treatment of AD. Apolipoprotein E, lipoprotein(a), oxidized LDL (low density lipoprotein)'s and large LDL particles, as well as other proinflammatory lipids and stress hormones such as cortisol, have been recognized as key factors in amyloid plaque formation and aggravation of AD. Extracorporeal lipoprotein apheresis systems employ well-established, powerful methods to provide an acute, reliable 60–80% reduction in the circulating concentration of these lipid classes and reduce acute cortisol levels. Following a double-membrane extracorporeal apheresis in patients with AD, there was a significant reduction of proinflammatory lipids, circulating cytokines, immune complexes, proinflammatory metals and toxic chaperones in patients with AD. On the basis of the above, we suggest designing clinical trials to assess the promising potential of such “cerebropheresis” treatment in patients with AD and, possibly, other neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fitz NF, Nam KN, Koldamova R, Lefterov I. Therapeutic targeting of nuclear receptors, LXR and RXR, for Alzheimer’s disease. Br J Pharmacol. 2019;176:3599–3610.
Ng TP, Feng L, Nyunt MS, Feng L, Gao Q, Lim ML, et al. Metabolic syndrome and the risk of mild cognitive impairment and progression to dementia: follow-up of the Singapore Longitudinal Ageing Study Cohort. JAMA Neurol. 2016;73:456–63.
Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23:174–84.
de la Monte SM, Tong M, Daiello LA, Ott BR. Early-stage Alzheimer’s disease is associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J Alzheimer's Dis. 2019;68:657–68.
Sharifipour E, Sharifimoghadam S, Hassanzadeh N, Ghasemian MN, Ghoreishi A, Hejazi SA, et al. Altered plasma visfatin levels and insulin resistance in patients with Alzheimer’s disease. Acta Neurol Belg. 2019. [Epub ahead of print].
Schally AV, Salgueiro L. Part III: Experimental studies on antagonists of LH-RH and GH-RH in animal models of Alzheimer’s disease: projections for treatment of other neurological conditions. Peptides. 2015;72:154–63.
Jaszberenyi M, Rick FG, Szalontay L, Block NL, Zarandi M, Cai RZ, et al. Beneficial effects of novel antagonists of GHRH in different models of Alzheimer’s disease. Aging. 2012;4:755–67.
Romero MJ, Lucas R, Dou H, Sridhar S, Czikora I, Mosieri EM, et al. Role of growth hormone-releasing hormone in dyslipidemia associated with experimental type 1 diabetes. Proc Natl Acad Sci USA. 2016;113:1895–1900.
Femminella GD, Frangou E, Love SB, Busza G, Holmes C, Ritchie C, et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials. 2019;20:191.
Batista AF, Bodart-Santos V, De Felice FG, Ferreira ST. Neuroprotective actions of glucagon-like peptide-1 (GLP-1) analogues in Alzheimer’s and Parkinson’s diseases. CNS Drugs. 2019;33:209–23.
Van DP, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci. 2018;12:930.
Dempsey C, Rubio AA, Bryson KJ, Finucane O, Larkin C, Mills EL, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61:306–16.
Button EB, Boyce GK, Wilkinson A, Stukas S, Hayat A, Fan J, et al. ApoA-I deficiency increases cortical amyloid deposition, cerebral amyloid angiopathy, cortical and hippocampal astrogliosis, and amyloid-associated astrocyte reactivity in APP/PS1 mice. Alzheimer's Res Ther. 2019;11:44.
Marais AD. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology. 2019;51:165–76.
Lee S, Parekh T, King SM, Reed B, Chui HC, Krauss RM, et al. Low-density lipoprotein particle size subfractions and cerebral amyloidosis. J Alzheimer's Dis. 2019;68:983–90.
Koch CA, Krabbe S, Hehmke B. Statins, metformin, proprotein-convertase-subtilisin-kexin type-9 (PCSK9) inhibitors and sex hormones: Immunomodulatory properties? Rev Endocr Metab Disord. 2018;19:363–95.
Koch CA, Antonelli A. Immunoendocrinology: when (neuro)endocrinology and immunology meet. Rev Endocr Metab Disord. 2018;19:277–82.
Mejias-Trueba M, Perez-Moreno MA, Fernandez-Arche MA. Systematic review of the efficacy of statins for the treatment of Alzheimer’s disease. Clin Med. 2018;18:54–61.
Leung YY, Toledo JB, Nefedov A, Polikar R, Raghavan N, Xie SX, et al. Identifying amyloid pathology-related cerebrospinal fluid biomarkers for Alzheimer’s disease in a multicohort study. Alzheimer's Dement. 2015;1:339–48.
Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–92.
Solfrizzi V, Panza F, D’Introno A, Colacicco AM, Capurso C, Basile AM, et al. Lipoprotein(a), apolipoprotein E genotype, and risk of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72:732–6.
Mielke MM, Haughey NJ, Han D, An Y, Bandaru VVR, Lyketsos CG, et al. The association between plasma ceramides and sphingomyelins and risk of Alzheimer’s disease differs by sex and APOE in the Baltimore Longitudinal Study of Aging. J Alzheimer's Dis. 2017;60:819–28.
Chatterjee P, Lim WL, Shui G, Gupta VB, James I, Fagan AM, et al. Plasma phospholipid and sphingolipid alterations in presenilin1 mutation carriers: a pilot study. J Alzheimer's Dis. 2016;50:887–94.
Panchal M, Gaudin M, Lazar AN, Salvati E, Rivals I, Ayciriex S, et al. Ceramides and sphingomyelinases in senile plaques. Neurobiol Dis. 2014;65:193–201.
Xing Y, Tang Y, Zhao L, Wang Q, Qin W, Zhang JL, et al. Plasma ceramides and neuropsychiatric symptoms of Alzheimer’s disease. J Alzheimer's Dis. 2016;52:1029–35.
Kim SH, Yang JS, Lee JC, Lee JY, Lee JY, Kim E, et al. Lipidomic alterations in lipoproteins of patients with mild cognitive impairment and Alzheimer’s disease by asymmetrical flow field-flow fractionation and nanoflow ultrahigh performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2018;1568:91–100.
Proitsi P, Kim M, Whiley L, Simmons A, Sattlecker M, Velayudhan L, et al. Association of blood lipids with Alzheimer’s disease: a comprehensive lipidomics analysis. Alzheimer's Dement. 2017;13:140–51.
Yu Q, He Z, Zubkov D, Huang S, Kurochkin I, Yang X, et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol Psychiatry. 2018. [Epub ahead of print].
Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27.
Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, et al. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry. 2008;165:1456–64.
Ouanes S, Castelao E, Gebreab S, von GA, Preisig M, Popp J. Life events, salivary cortisol, and cognitive performance in nondemented subjects: a population-based study. Neurobiol Aging. 2017;51:1–8.
Sang YM, Wang LJ, Mao HX, Lou XY, Zhu YJ. The association of short-term memory and cognitive impairment with ghrelin, leptin, and cortisol levels in non-diabetic and diabetic elderly individuals. Acta Diabetol. 2018;55:531–9.
Ouanes S, Popp J. High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front Aging Neurosci. 2019;11:43.
Popp J, Wolfsgruber S, Heuser I, Peters O, Hull M, Schroder J, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36:601–7.
Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T, et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry. 2013;18:993–1005.
Moreno-Jimenez EP, Flor-Garcia M, Terreros-Roncal J, Rabano A, Cafini F, Pallas-Bazarra N, et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med. 2019;25:554–60.
Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–39.
Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–43.
Steenblock C, Rubin de Celis MF, Delgadillo Silva LF, Pawolski V, Brennand A, Werdermann M, et al. Isolation and characterization of adrenocortical progenitors involved in the adaptation to stress. Proc Natl Acad Sci USA. 2018;115:12997–3002.
Bornstein SR, Steenblock C, Chrousos GP, Schally AV, Beuschlein F, Kline G, et al. Stress-inducible-stem cells: a new view on endocrine, metabolic and mental disease? Mol Psychiatry. 2019;24:2–9.
Schettler VJJ, Neumann CL, Peter C, Zimmermann T, Julius U, Hohenstein B, et al. Lipoprotein apheresis is an optimal therapeutic option to reduce increased Lp(a) levels. Clin Res Cardiol Suppl. 2019;14(Suppl 1):33–38.
Leebmann J, Roeseler E, Julius U, Heigl F, Spitthoever R, Heutling D, et al. Lipoprotein apheresis in patients with maximally tolerated lipid-lowering therapy, lipoprotein(a)-hyperlipoproteinemia, and progressive cardiovascular disease: prospective observational multicenter study. Circulation. 2013;128:2567–76.
Khan TZ, Hsu LY, Arai AE, Rhodes S, Pottle A, Wage R, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J. 2017;38:1561–9.
Straube R, Voit-Bak K, Gor A, Steinmeier T, Chrousos GP, Boehm BO, et al. Lipid profiles in lyme borreliosis: a potential role for apheresis? Horm Metab Res. 2019;51:326–9.
Morawietz H, Goettsch W, Brux M, Reimann M, Bornstein SR, Julius U, et al. Lipoprotein apheresis of hypercholesterolemic patients mediates vasoprotective gene expression in human endothelial cells. Atheroscler Suppl. 2013;14:107–13.
Orsoni A, Saheb S, Levels JH, Dallinga-Thie G, Atassi M, Bittar R, et al. LDL-apheresis depletes apoE-HDL and pre-beta1-HDL in familial hypercholesterolemia: relevance to atheroprotection. J Lipid Res. 2011;52:2304–13.
Schettler VJ, Muellendorff F, Schettler E, Platzer C, Norkauer S, Julius U, et al. NMR-based lipoprotein analysis for patients with severe hypercholesterolemia undergoing lipoprotein apheresis or PCSK9-inhibitor therapy (NAPALI-Study). Ther Apher Dial. 2019. [Epub ahead of print].
Lappegard KT, Kjellmo CA, Ljunggren S, Cederbrant K, Marcusson-Stahl M, Mathisen M, et al. Lipoprotein apheresis affects lipoprotein particle subclasses more efficiently compared to the PCSK9 inhibitor evolocumab, a pilot study. Transfus Apher Sci. 2018;57:91–96.
Kopprasch S, Bornstein SR, Bergmann S, Graessler J, Hohenstein B, Julius U. Long-term follow-up of circulating oxidative stress markers in patients undergoing lipoprotein apheresis by Direct Adsorption of Lipids (DALI). Atheroscler Suppl. 2017;30:115–21.
Kopprasch S, Bornstein SR, Bergmann S, Graessler J, Julius U. Long-term therapeutic efficacy of lipoprotein apheresis on circulating oxidative stress parameters—a comparison of two different apheresis techniques. Atheroscler Suppl. 2015;18:80–84.
Tselmin S, Schmitz G, Julius U, Bornstein SR, Barthel A, Graessler J. Acute effects of lipid apheresis on human serum lipidome. Atheroscler Suppl. 2009;10:27–33.
Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry. 2013;74:357–66.
Lesuis SL, Weggen S, Baches S, Lucassen PJ, Krugers HJ. Targeting glucocorticoid receptors prevents the effects of early life stress on amyloid pathology and cognitive performance in APP/PS1 mice. Transl Psychiatry. 2018;8:53.
Webster SP, McBride A, Binnie M, Sooy K, Seckl JR, Andrew R, et al. Selection and early clinical evaluation of the brain-penetrant 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitor UE2343 (Xanamem). Br J Pharm. 2017;174:396–408.
Walther R, Julius U, Tselmin S, Schatz U, Bornstein SR, Graessler J. Short- and long-term effects of lipoprotein apheresis on plasma hormones in patients with therapy-resistant dyslipidemia. Atheroscler Suppl. 2019. [Epub ahead of print].
Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136(Suppl 1):29–38.
Guedes JR, Lao T, Cardoso AL, El KJ. Roles of microglial and monocyte chemokines and their receptors in regulating Alzheimer’s disease-associated amyloid-beta and tau pathologies. Front Neurol. 2018;9:549.
Haskins M, Jones TE, Lu Q, Bareiss SK. Early alterations in blood and brain RANTES and MCP-1 expression and the effect of exercise frequency in the 3xTg-AD mouse model of Alzheimer’s disease. Neurosci Lett. 2016;610:165–70.
Cameron B, Landreth GE. Inflammation, microglia, and Alzheimer’s disease. Neurobiol Dis. 2010;37:503–9.
Ravindran D, Ridiandries A, Vanags LZ, Henriquez R, Cartland S, Tan JT, et al. Chemokine binding protein ‘M3’ limits atherosclerosis in apolipoprotein E-/- mice. PLoS ONE. 2017;12:e0173224.
Feng X, Gao X, Jia Y, Zhang H, Xu Y. Atorvastatin decreased circulating RANTES levels in impaired glucose tolerance patients with hypercholesterolemia: an interventional study. Diabetes Ther. 2017;8:309–19.
Reale M, Kamal MA, Velluto L, Gambi D, Di NM, Greig NH. Relationship between inflammatory mediators, Abeta levels and ApoE genotype in Alzheimer disease. Curr Alzheimer Res. 2012;9:447–57.
Seddighi S, Varma V, Thambisetty M. alpha2-macroglobulin in Alzheimer’s disease: new roles for an old chaperone. Biomark Med. 2018;12:311–4.
Tomljenovic L. Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimer's Dis. 2011;23:567–98.
Meleleo D, Notarachille G, Mangini V, Arnesano F. Concentration-dependent effects of mercury and lead on Abeta42: possible implications for Alzheimer’s disease. Eur Biophys J. 2019;48:173–87.
Russ TC, Killin LOJ, Hannah J, Batty GD, Deary IJ, Starr JM. Aluminium and fluoride in drinking water in relation to later dementia risk. Br J Psychiatry. 2019:1–6. [Epub ahead of print].
Mostafalou S, Abdollahi M. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology. 2018;409:44–52.
Boada M, Ramos-Fernandez E, Guivernau B, Munoz FJ, Costa M, Ortiz AM, et al. Treatment of Alzheimer disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: rationale and treatment approach of the AMBAR (Alzheimer Management By Albumin Replacement) study. Neurologia. 2016;31:473–81.
Kawaguchi K, Saigusa A, Yamada S, Gotoh T, Nakai S, Hiki Y, et al. Toward the treatment for Alzheimer’s disease: adsorption is primary mechanism of removing amyloid beta protein with hollow-fiber dialyzers of the suitable materials, polysulfone and polymethyl methacrylate. J Artif Organs. 2016;19:149–58.
Acknowledgements
This work was supported by Transcampus and the Deutsche Forschungsgemeinschaft (GRK 2251/1, TRR 205/1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests in relation to the work. KVB, RB work for the INUS clinic and SRB is a consultant.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bornstein, S.R., Voit-Bak, K., Rosenthal, P. et al. Extracorporeal apheresis therapy for Alzheimer disease—targeting lipids, stress, and inflammation. Mol Psychiatry 25, 275–282 (2020). https://doi.org/10.1038/s41380-019-0542-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0542-x
This article is cited by
-
Navigating the metabolic maze: anomalies in fatty acid and cholesterol processes in Alzheimer’s astrocytes
Alzheimer's Research & Therapy (2024)
-
Chronic post-COVID-19 syndrome and chronic fatigue syndrome: Is there a role for extracorporeal apheresis?
Molecular Psychiatry (2022)
-
Liposome-based targeting of dopamine to the brain: a novel approach for the treatment of Parkinson’s disease
Molecular Psychiatry (2021)
-
Effects of safflower yellow on cholesterol levels in serum and brain tissue of APP/PS1 mice
Metabolic Brain Disease (2021)