Abstract
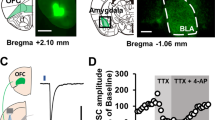
Stress can precipitate or worsen symptoms of many psychiatric disorders by dysregulating glutamatergic function within the prefrontal cortex (PFC). Previous studies suggest that antagonists of group II metabotropic glutamate (mGlu) receptors (mGlu2 and mGlu3) reduce stress-induced anhedonia through actions in the PFC, but the mechanisms by which these receptors act are not known. We now report that activation of mGlu3 induces long-term depression (LTD) of excitatory transmission in the PFC at inputs from the basolateral amygdala. Our data suggest mGlu3-LTD is mediated by postsynaptic AMPAR internalization in PFC pyramidal cells, and we observed a profound impairment in mGlu3-LTD following a single, 20-min restraint stress exposure. Finally, blocking mGlu3 activation in vivo prevented the stress-induced maladaptive changes to amydalo-cortical physiology and motivated behavior. These data demonstrate that mGlu3 mediates stress-induced physiological and behavioral impairments and further support the potential for mGlu3 modulation as a treatment for stress-related psychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;26:148.
Arnsten AFT, Raskind MA, Taylor FB, Connor DF. The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress. 2015;1:89–99.
Ossewaarde L, Qin S, Van Marle HJF, van Wingen GA, Fernández G, Hermans EJ. Stress-induced reduction in reward-related prefrontal cortex function. Neuroimage. 2011;55:345–52.
Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32.
Caetano SC, Kaur S, Brambilla P, Nicoletti M, Hatch JP, Sassi RB, et al. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2006;59:702–6.
Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–7.
Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–98.
Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–64.
Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–41.
Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33:773–83.
Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79.
Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–77.
Anderson AK. Feeling emotional: the amygdala links emotional perception and experience. Soc Cogn Affect Neurosci. 2007;2:71–72.
Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JNP, Monyer H, et al. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 2014;15:181–92.
Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal–prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–14.
Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci. 2017;20:824–35.
Otis JM, Namboodiri VMK, Matan AM, Voets ES, Mohorn EP, Kosyk O, et al. Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature. 2017;543:103–7.
Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13:251–66.
Chaki S. MGlu2/3 receptor antagonists as novel antidepressants. Trends Pharmacol Sci. 2017;38:569–80.
Bishop JR, Ellingrod VL, Moline J, Miller D. Association between the polymorphic GRM3 gene and negative symptom improvement during olanzapine treatment. Schizophr Res. 2005;77:253–60.
Harrison PJ, Lyon L, Sartorius LJ, Burnet PWJ, Lane TT. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–22.
Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT et al. mGluR2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: postsynaptic mGluR3 strengthen working memory networks. Cereb Cortex. 2017. https://doi.org/10.1093/cercor/bhx005.
Lainiola M, Procaccini C, Linden AM. MGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav Brain Res. 2014;266:94–103.
Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, et al. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci USA. 2015;112:1196–201.
Otani S, Daniel H, Takita M, Crépel F. Long-term depression induced by postsynaptic group II metabotropic glutamate receptors linked to phospholipase C and intracellular calcium rises in rat prefrontal cortex. J Neurosci. 2002;22:3434–44.
Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–68.
Joffe ME, Grueter BA. Cocaine experience enhances thalamo-accumbens N-Methyl-D-Aspartate receptor function. Biol Psychiatry. 2016;80:671–81.
Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, et al. Role for the M1 muscarinic acetylcholine receptor in top-down cognitive processing using a touchscreen visual discrimination task in mice. ACS Chem Neurosci. 2015;6:1683–95.
Jin LE, Wang M, Yang S-T, Yang Y, Galvin VC, Lightbourne TC, et al. mGluR2/3 mechanisms in primate dorsolateral prefrontal cortex: evidence for both presynaptic and postsynaptic actions. Mol Psychiatry. 2016;22:1615–25.
Huang CC, Yang PC, Lin HJ, Hsu KS. Repeated cocaine administration impairs group II metabotropic glutamate receptor-mediated long-term depression in rat medial prefrontal cortex. J Neurosci. 2007;27:2958–68.
Engers JL, Rodriguez AL, Konkol LC, Morrison RD, Thompson AD, Byers FW, et al. Discovery of a selective and CNS penetrant negative allosteric modulator of metabotropic glutamate receptor subtype 3 with antidepressant and anxiolytic activity in rodents. J Med Chem. 2015;58:7485–7500.
Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–25.
Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–58.
Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–25.
Porcelli AJ, Delgado MR. Stress and decision making: effects on valuation, learning, and risk-taking. Curr Opin Behav Sci. 2017;14:33–39.
Izquierdo A, Wellman C, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–8.
Maier SU, Makwana AB, Hare TA. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain’s decision circuits. Neuron. 2015;87:621–31.
Olausson P, Kiraly DD, Gourley SL, Taylor JR. Persistent effects of prior chronic exposure to corticosterone on reward-related learning and motivation in rodents. Psychopharmacology. 2013;225:569–77.
Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–34.
Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322.
Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–41.
Robbe D, Bockaert J, Manzoni OJ. Metabotropic glutamate receptor 2/3-dependent long-term depression in the nucleus accumbens is blocked in morphine withdrawn mice. Eur J Neurosci. 2002;16:2231–5.
Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–83.
Johnson KA, Lovinger DM. Presynaptic G protein-coupled receptors: gatekeepers of addiction? Front Cell Neurosci. 2016;10:264.
Rosenberg N, Gerber U, Ster J. Activation of group II metabotropic glutamate receptors promotes LTP induction at schaffer collateral-CA1 pyramidal cell synapses by priming NMDA receptors. J Neurosci. 2016;36:11521–31.
Trepanier C, Lei G, Xie Y-F, MacDonald JF. Group II metabotropic glutamate receptors modify N-methyl-D-aspartate receptors via Src kinase. Sci Rep. 2013;3:926.
Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–44.
Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–59.
Lucas SJ, Bortolotto ZA, Collingridge GL, Lodge D. Selective activation of either mGlu2 or mGlu3 receptors can induce LTD in the amygdala. Neuropharmacology. 2013;66:196–201.
Han JS, Bird GC, Neugebauer V. Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Neuropharmacology. 2004;46:918–26.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2011;73:962–77.
Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen B, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–9.
Pittenger C, Duman RS, Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109.
Bagley J, Moghaddam B, Haven W. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73.
Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5-HT neurons in the DRN. Neuropsychopharmacology. 2015;41:1046–56.
Koike H, Iijima M, Chaki S. Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav. 2013;107:20–23.
Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Neurospcyhopharmacology. 2012;15:429–34.
Dwyer JM, Lepack AE, Duman RS. mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry. 2013;1:15.
Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol. 2015;172:4254–76.
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. 2016;89:857–66.
Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209.
Selden NRW, Everitt BJ, Jarrard LE, Robbins TW. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:335–50.
Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85.
Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal Ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. J Neurosci. 2014;34:8462–6.
Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, et al. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: role of ΔFosB. J Neurosci. 2014;34:3878–87.
Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–84.
Jenni NL, Larkin JD, Floresco SB. Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of risk/reward decision-making via distinct ventral striatal and amygdalar circuits output from. J Neurosci. 2012;37:6200–13.
St. Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–33.
Land BB, Narayanan NS, Liu R-J, Gianessi CA, Brayton CE, Grimaldi DM, et al. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–53.
Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–7.
Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–7.
Mcomish CE, Pavey G, Gibbons A, Hopper S, Udawela M, Scarr E, et al. Lower [3H]LY341495 binding to mGlu2/3 receptors in the anterior cingulate of subjects with major depressive disorder but not bipolar disorder or schizophrenia. J Affect Disord. 2016;190:241–8.
Kasanetz F, Manzoni OJ. Maturation of excitatory synaptic transmission of the rat nucleus accumbens from juvenile to adult. J Neurophysiol. 2009;101:2516–27.
Joffe ME, Grueter CA, Grueter BA. Biological substrates of addiction. Wiley Interdiscip Rev Cogn Sci. 2014;5:151–71.
Acknowledgements
The Authors thank Douglas Shaw, Jennifer Zachry, Weimin Peng, and Dr. Zixiu Xiang for their technical assistance and advice. This work was supported by the National Institutes of Health (NIH) grants R01MH062646 and R37NS031373 (P.J.C.). M.E.J. was supported by the NIH grant T32MH093366 and a postdoctoral fellowship through the Pharmaceutical Research and Manufacturers of America Foundation. Behavioral experiments were performed through the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.W.L. has been funded by the NIH, Johnson and Johnson, Bristol-Myers Squibb, AstraZeneca, Michael J. Fox Foundation, as well as Seaside Therapeutics. He has consulted for AbbVie and received compensation. P.J.C. has been funded by NIH, AstraZeneca, Bristol-Myers Squibb, Michael J. Fox Foundation, Dystonia Medical Research Foundation, CHDI Foundation, and Thome Memorial Foundation. Over the past 3 years he has served on the Scientific Advisory Boards for Michael J. Fox Foundation, Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and Neurobiology Foundation for Schizophrenia and Bipolar Disorder. C.W.L. and P.J.C. are inventors on patents that protect different classes of metabotropic glutamate allosteric modulators. M.E.J., C.I.S., and J.L.E. declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Joffe, M.E., Santiago, C.I., Engers, J.L. et al. Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry 24, 916–927 (2019). https://doi.org/10.1038/s41380-017-0015-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-017-0015-z
This article is cited by
-
Are mGluR2/3 Inhibitors Potential Compounds for Novel Antidepressants?
Cellular and Molecular Neurobiology (2023)
-
The genie in the bottle-magnified calcium signaling in dorsolateral prefrontal cortex
Molecular Psychiatry (2021)
-
A system biology approach based on metabolic biomarkers and protein–protein interactions for identifying pathways underlying schizophrenia and bipolar disorder
Scientific Reports (2021)
-
Frontal cortex genetic ablation of metabotropic glutamate receptor subtype 3 (mGlu3) impairs postsynaptic plasticity and modulates affective behaviors
Neuropsychopharmacology (2021)
-
Antidepressant potential of metabotropic glutamate receptor mGlu2 and mGlu3 negative allosteric modulators
Neuropsychopharmacology (2019)