Abstract
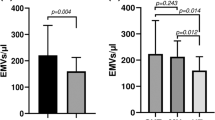
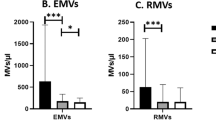
Psoriasis is associated with increased cardiovascular risk. Endothelial, platelet, and erythrocyte microvesicles (MVs) are novel biomarkers of endothelial dysfunction and thromboinflammation. We explored whether MVs of different cell types are elevated in patients with psoriasis, and investigated potential associations with disease severity and macrovascular function. Endothelial, platelet and erythrocyte MVs were measured using a standardized flow cytometry protocol in psoriasis patients and controls free from established cardiovascular disease. Carotid intima-media thickness (IMT) and pulse wave velocity (PWV) were measured as markers of subclinical atherosclerosis and arterial stiffness. Psoriasis severity was assessed with PASI (Psoriasis Area Severity Index). Both platelet (p < 0.001) and erythrocyte MVs (p = 0.046), yet not endothelial MVs, were significantly increased in patients with psoriasis (n = 41) compared with controls (n = 41). Patients with higher PASI (≥10) presented significantly higher levels of ErMVs compared to those with lower PASI (<10) (p = 0.047). Carotid IMT and PWV were comparable between psoriasis patients and controls and did not significantly correlate with MVs. In the multivariate analysis, psoriasis was identified as an independent predictor of both platelet (p < 0.001) and erythrocyte MVs (p = 0.043), while hypertension was independently associated with endothelial MVs (p < 0.001). Increased formation of platelet and erythrocyte MVs may be evident in psoriasis patients and is indicative of prothrombotic, proinflammatory microenvironment, even in the absence of subclinical macrovascular dysfunction and before the clinical onset of overt cardiovascular complications. Potential mechanistic links and prognostic implications of increased MVs in psoriasis warrant further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
Data available upon reasonable request from the corresponding author.
References
Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983–94.
Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. 2020;37:2017–33.
Anyfanti P, Gavriilaki E, Douma S, Gkaliagkousi E. Endothelial dysfunction in patients with rheumatoid arthritis: the role of hypertension. Curr Hypertens Rep. 2020;22:56.
Ekdahl K, Teramura Y, Asif S, Jonsson N, Magnusson P, Nilsson B. Thromboinflammation in therapeutic medicine. Adv Exp Med Biol. 2015;865:3–17.
Aksu K, Donmez A, Keser G. Inflammation-induced management thrombosis: mechanisms, disease associations and management. Curr Pharm Des. 2012;18:1478–93.
Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat M-L, et al. Microvesicles in vascular homeostasis and diseases. Thromb Haemost. 2017;117:1296–316.
Lipets EN, Antonova OA, Shustova ON, Losenkova KV, Mazurov AV, Ataullakhanov FI. Use of Thrombodynamics for revealing the participation of platelet, erythrocyte, endothelial, and monocyte microparticles in coagulation activation and propagation. PLoS One. 2020;15:e0227932.
Noubouossie DF, Henderson MW, Mooberry M, Ilich A, Ellsworth P, Piegore M, et al. Red blood cell microvesicles activate the contact system, leading to factor IX activation via 2 independent pathways. Blood. 2020;135:755–65.
Gkaliagkousi E, Gavriilaki E, Vasileiadis I, Nikolaidou B, Yiannaki E, Lazaridis A, et al. Endothelial microvesicles circulating in peripheral and coronary circulation are associated with central blood pressure in coronary artery disease. Am J Hypertens. 2019;32:1199–205.
Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, et al. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diabetes Vasc Dis Res. 2019;16:458–65.
Gkaliagkousi E, Gavriilaki E, Yiannaki E, Vasileiadis I, Nikolaidou B, Lazaridis A, et al. Platelet microvesicles are associated with the severity of coronary artery disease: comparison between peripheral and coronary circulation. J Thromb Thrombolysis. https://doi.org/10.1007/s11239-020-02302-5.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens. 2018;36:1953–2041.
Report and Consultation (WHO) WHO. Abbreviated report of a WHO consultation. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. 2011; 1–25.
Consultation. Definition and diagnosis of diabetes and intermediate hyperglycemia. World Health Organization (WHO). 2006.
World Medical Association Declaration of Helsinki. JAMA. 2013;310:2191.
Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular. J Am Soc Echocardiogr. 2008;21:93–111.
Harada A, Okada T, Niki K, Chang D, Sugawara M. On-line noninvasive one-point measurements of pulse wave velocity. Heart Vessels. 2002;17:61–68.
Lin H-F, Liu C-K, Liao Y-C, Lin R-T, Chen C-S, Juo S-HH. The risk of the metabolic syndrome on carotid thickness and stiffness: sex and age specific effects. Atherosclerosis. 2010;210:155–9.
Suresh K, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13.
Pelletier F, Garnache-Ottou F, Angelot F, Biichlé S, Vidal C, Humbert P, et al. Increased levels of circulating endothelial-derived microparticles and small-size platelet-derived microparticles in psoriasis. J Investig Dermatol. 2011;131:1573–6.
Späh F. Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159:10–17.
Boehncke WH. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front Immunol. 2018;9:579.
Frangou E, Chrysanthopoulou A, Mitsios A, Kambas K, Arelaki S, Angelidou I, et al. REDD1_autophagy pathway promotes thromboinflammation and fibrosis in human systemic lupus erythematosus (SLE). Ann Rheum Dis. 2019;78:238–48.
Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2013;73:1854–63.
Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–802.
Anyfanti P, Gavriilaki E, Nikolaidou B, Yiannaki E, Lazaridis A, Papadopoulos N, et al. Patients with autoimmune chronic inflammatory diseases present increased biomarkers of thromboinflammation and endothelial dysfunction in the absence of flares and cardiovascular comorbidities. J Thromb Thrombolysis. 2022;53:10–6.
Garshick MS, Tawil M, Barrett TJ, Salud-Gnilo CM, Eppler M, Lee A, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol. 2020;40:1340–51.
Herster F, Bittner Z, Codrea MC, Archer NK, Heister M, Löffler MW, et al. Platelets aggregate with neutrophils and promote skin pathology in psoriasis. Front Immunol. 2019;10:1867.
Herster F, Karbach S, Chatterjee M, Weber ANR. Platelets: underestimated regulators of autoinflammation in psoriasis. J Investig Dermatol. https://doi.org/10.1016/j.jid.2020.12.025 2021.
Martínez-Sales V, Vila V, Ricart JM, Vayá A, Todolí J, Nñez C, et al. Increased circulating endothelial cells and microparticles in patients with psoriasis. Clin Hemorheol Microcirc. 2015;60:283–90.
Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: Increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol. 2010;62:621–6.
Papadavid E, Diamanti K, Spathis A, Varoudi M, Andreadou I, Gravanis K, et al. Increased levels of circulating platelet-derived microparticles in psoriasis: Possible implications for the associated cardiovascular risk. World J Cardiol. 2016;8:667.
Takeshita J, Mohler ER, Krishnamoorthy P, Moore J, Rogers WT, Zhang L, et al. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc. 2014;3:1–8.
Leal JKF, Adjobo-Hermans MJW, Bosman GJCGM. Red blood cell homeostasis: mechanisms and effects of microvesicle generation in health and disease. Front Physiol. 2018; 9. https://doi.org/10.3389/fphys.2018.00703.
Lazaridis A, Gavriilaki E, Nikolaidou B, Yiannaki E, Dolgyras P, Anyfanti P, et al. A study of endothelial and platelet microvesicles across different hypertension phenotypes. J Hum Hypertens. 2021. https://doi.org/10.1038/s41371-021-00531-6.
Gkaliagkousi E, Gavriilaki E, Triantafyllou A, Douma S. Clinical significance of endothelial dysfunction in essential hypertension. Curr Hypertens Rep. 2015; 17. https://doi.org/10.1007/s11906-015-0596-3.
Funding
P.A., E. Gavriilaki and E. Gkaliagkousi are funded by European Social Fund- ESF through the Operational Program “Human Resources Development, Education and Lifelong Learning 2014-2020” in the context of the project “Evaluation of novel markers of endothelial dysfunction and thrombotic microenvironment in patients with rheumatoid arthritis: association with markers of subclinical inflammation and cardiovascular damage (MIS 5047870)”.
Author information
Authors and Affiliations
Contributions
Conceptualization, EG (Eugenia Gkaliagkousi) and SD; methodology, EG (Eugenia Gkaliagkousi) and AP; validation, AL, and BN; formal analysis, PA; investigation, AM, EY, and AL; data curation, EG (Eleni Gavriilaki) and AT; writing—original draft preparation, KG, AM, and EG (Eleni Gavriilaki); writing—review and editing, PA; supervision, EG (Eugenia Gkaliagkousi) and EL; funding acquisition, EG (Eugenia Gkaliagkousi) and SD.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study was conducted in accordance with the Helsinki declaration and was approved by the institutional ethics committee. Written informed consent was provided before inclusion in the study.
Informed consent
Written informed consent was provided before inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Margouta, A., Anyfanti, P., Lazaridis, A. et al. Assessment of microvesicles from different cell origins in patients with psoriasis: evidence of thrombogenic, proinflammatory microenvironment in the absence of established cardiovascular disease. J Hum Hypertens 37, 925–930 (2023). https://doi.org/10.1038/s41371-022-00787-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-022-00787-6