Abstract
Background/objective
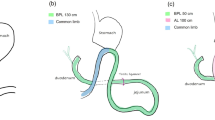
Biliopancreatic diversion with duodenal switch (BPD-DS) is the most effective bariatric intervention to treat morbid obesity and related disorders. Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) is a new bariatric procedure devised with the purpose of simplifying the complexity of the BPD-DS technique while maintaining its efficacy. However, whether BPD-DS and SADI-S result in similar fasting and post-prandial hormone profiles has not yet been studied. Therefore, the purpose of this study was to assess and compare the hormone response to a standardized mixed meal in subjects operated with BPD-DS or SADI-S.
Subjects/methods
Subjects submitted to BPD-DS (n = 9) or SADI-S (n = 9) 1.5 years earlier on average, with no past nor current diabetes diagnosis underwent a liquid mixed-meal tolerance test (MMTT) to assess the baseline and post-prandial profile of glucose, enteropancreatic hormones and total bile acids.
Results
Fasting glucose, enteropancreatic hormones and total bile acids levels after BPD-DS and SADI-S were similar. After the MMTT, the response of subjects who underwent SADI-S was characterized by higher glucose (t = 30 min: p < 0.05; iAUC: 156.1 ± 46.2 vs. 103.4 ± 35.8 mmol/L × min, p = 0.02), GLP-1 (t = 30 min: p < 0.05; iAUC: 5388 ± 3010 vs. 2959.0 ± 2146 pmol/L × min, p = 0.02), glucagon (t = 30 min: p < 0.05; iAUC: 678.7 ± 295.2 vs. 376.9 ± 215.7 pmol/L × min, p = 0.02), insulin (t = 30 and 45 min: p < 0.05); and C-peptide levels (t = 30 and 45 min: p < 0.05), when compared to BPD-DS.
Conclusions
The post-prandial hormone secretion profile after SADI-S is characterized by increased GLP-1, glucagon and insulin secretion, when compared to BPD-DS, which suggests the existence of different endocrine driven mechanisms leading to weight loss and metabolic improvement after the two procedures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Skogar ML, Sundbom M. Duodenal switch is superior to gastric bypass in patients with super obesity when evaluated with the Bariatric Analysis and Reporting Outcome System (BAROS). Obes Surg. 2017;27:2308–16.
Strain GW, Torghabeh MH, Gagner M, Ebel F, Dakin GF, Abelson JS, et al. The Impact of Biliopancreatic Diversion with Duodenal Switch (BPD/DS) over 9 years. Obes Surg. 2017;27:787–94.
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Sanchez-Pernaute A, Rubio Herrera MA, Perez-Aguirre E, Garcia Perez JC, Cabrerizo L, Diez Valladares L, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17:1614–8.
Torres A, Rubio MA, Ramos-Levi AM, Sanchez-Pernaute A. Cardiovascular risk factors after Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S): a New Effective Therapeutic Approach? Curr Atheroscler Rep. 2017;19:58.
Cottam A, Cottam D, Portenier D, Zaveri H, Surve A, Cottam S, et al. A matched cohort analysis of Stomach Intestinal Pylorus Saving (SIPS) surgery versus biliopancreatic diversion with duodenal switch with two-year follow-up. Obes Surg. 2017;27:454–61.
Surve A, Zaveri H, Cottam D, Belnap L, Cottam A, Cottam S. A retrospective comparison of biliopancreatic diversion with duodenal switch with single anastomosis duodenal switch (SIPS-stomach intestinal pylorus sparing surgery) at a single institution with two year follow-up. Surg Obes Relat Dis. 2017;13:415–22.
Cottam A, Cottam D, Zaveri H, Cottam S, Surve A, Medlin W, et al. An analysis of mid-term complications, weight loss, and type 2 diabetes resolution of Stomach Intestinal Pylorus-Sparing Surgery (SIPS) Versus Roux-En-Y Gastric Bypass (RYGB) with Three-Year Follow-Up. Obes Surg. 2018;28:2894–902.
Surve A, Cottam D, Sanchez-Pernaute A, Torres A, Roller J, Kwon Y, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;14:594–601.
Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43:535–9.
Patricio BG, Morais T, Guimaraes M, Veedfald S, Hartmann B, Hilsted L, et al. Gut hormone release after gastric bypass depends on the length of the biliopancreatic limb. Int J Obes. 2018.
Krarup T, Holst JJ. The heterogeneity of gastric inhibitory polypeptide in porcine and human gastrointestinal mucosa evaluated with five different antisera. Regul Pept. 1984;9:35–46.
Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87:415–23.
Toräng S, Bojsen-Moller KN, Svane MS, Hartmann B, Rosenkilde MM, Madsbad S, et al. In vivo and in vitro degradation of peptide YY3-36 to inactive peptide YY3-34 in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310:R866–74.
Kuhre RE, Bechmann LE, Wewer Albrechtsen NJ, Hartmann B, Holst JJ. Glucose stimulates neurotensin secretion from the rat small intestine by mechanisms involving SGLT1 and GLUT2, leading to cell depolarization and calcium influx. Am J Physiol Endocrinol Metab. 2015;308:E1123–30.
Pedersen JH, Stadil F, Fahrenkrug J. Preparation of 125I-(Tyr 3)- and 125I-(Tyr 11)- neurotensin for radioimmunoassay. Scand J Clin Lab Invest. 1983;43:483–91.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Prog Biomed. 1996;50:253–64.
Gropper SS, Smith JL. Advanced nutrition and human metabolism: Cengage Learning; 2012.
Torekov SS, Iepsen E, Christiansen M, Linneberg A, Pedersen O, Holst JJ, et al. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes. 2014;63:1315–25.
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601.
Palha AM, Pereira SS, Costa MM, Morais T, Maia AF, Guimaraes M, et al. Differential GIP/GLP-1 intestinal cell distribution in diabetics’ yields distinctive rearrangements depending on Roux-en-Y biliopancreatic limb length. J Cell Biochem. 2018;119:7506–14.
Guedes TP, Martins S, Costa M, Pereira SS, Morais T, Santos A, et al. Detailed characterization of incretin cell distribution along the human small intestine. Surg Obes Relat Dis. 2015;11:1323–31.
Kuhre RE, Wewer Albrechtsen NJ, Larsen O, Jepsen SL, Balk-Moller E, Andersen DB, et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol Metab. 2018;11:84–95.
Ullmer C, Alvarez Sanchez R, Sprecher U, Raab S, Mattei P, Dehmlow H, et al. Systemic bile acid sensing by G protein-coupled bile acid receptor 1 (GPBAR1) promotes PYY and GLP-1 release. Br J Pharmacol. 2013;169:671–84.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–77.
Adrian TE, Ballantyne GH, Longo WE, Bilchik AJ, Graham S, Basson MD, et al. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–24.
Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–35.
Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grondahl M, Hartmann B, et al. Evidence of Extrapancreatic Glucagon Secretion in Man. Diabetes. 2016;65:585–97.
Holst JJ, Pedersen JH, Baldissera F, Stadil F. Circulating glucagon after total pancreatectomy in man. Diabetologia. 1983;25:396–9.
Vilsboll T. The effects of glucagon-like peptide-1 on the beta cell. Diabetes Obes Metab. 2009;11(Suppl 3):11–8.
MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(suppl 3):S434–S42.
Li J, Song J, Zaytseva YY, Liu Y, Rychahou P, Jiang K, et al. An obligatory role for neurotensin in high-fat-diet-induced obesity. Nature. 2016;533:411–5.
Ratner C, Hundahl C, Holst B. The metabolic actions of neurotensin secreted from the gut. Cardiovasc Endocrinol & Metab. 2016;5:102–11.
von Loeffelholz C, Gissey LC, Schumann T, Henke C, Kurzbach A, Struck J, et al. The anorexigenic peptide neurotensin relates to insulin sensitivity in obese patients after BPD or RYGB metabolic surgery. Int J Obes. 2018.
Christ-Crain M, Stoeckli R, Ernst A, Morgenthaler NG, Bilz S, Korbonits M, et al. Effect of gastric bypass and gastric banding on proneurotensin levels in morbidly obese patients. J Clin Endocrinol Metab. 2006;91:3544–7.
Tymitz K, Engel A, McDonough S, Hendy MP, Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes Surg. 2011;21:125–30.
Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96:201–26.
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–9.
Festa A, Williams K, D’Agostino R Jr., Wagenknecht LE, Haffner SM. The natural course of beta-cell function in nondiabetic and diabetic individuals: the insulin resistance atherosclerosis study. Diabetes. 2006;55:1114–20.
Fonseca VA. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S151–6.
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35:1605–10.
Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95.
Acknowledgements
Authors would like to thank the Senior Nurse Sandra Tavares (Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal) for her technical assistance during the MMTT; and Lene Brus Albaek (Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark), and Lene Ravn (Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark) for their technical assistance with assays.
Funding:
UMIB is funded by grants from Foundation for Science and Technology (FCT) Portugal (UID/ Multi/00215/2013 and UID/MULTI/0215/2016). JJH holds an unrestricted grant from the NNF Center for Basic Metabolic Research, Copenhagen, Denmark. The NNF Center for Basic Metabolic Research is an independent research institution at the University of Copenhagen, Denmark.
Author contributions:
Conceived and designed the experiments: MG, MN, JJH, MPM; performed the experiments: SSP, MG, RA, AMP, CBL, BH, MN, LH; analyzed data: SSP, CBL, BH; contributed reagents/materials/analysis tools: LH, JJH, MN, MMP; discussed the results and implications: SSP, MG, MN, JJH, MPM; wrote or reviewed the paper: SSP, MG, RA, AMP, CBL, BH, LH, JJH, MN, MPM. All authors have approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pereira, S.S., Guimarães, M., Almeida, R. et al. Biliopancreatic diversion with duodenal switch (BPD-DS) and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) result in distinct post-prandial hormone profiles. Int J Obes 43, 2518–2527 (2019). https://doi.org/10.1038/s41366-018-0282-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0282-z
This article is cited by
-
One-Stage Vs. Two-Step One Anastomosis Duodenal Switch (OADS/SADI-S): A Safety and Efficacy Single-Center Propensity-Score Matched Analysis
Obesity Surgery (2024)
-
Remission of Type II Diabetes Mellitus after Duodenal Switch: the Contribution of Common Channel Length
Obesity Surgery (2023)
-
Towards precision medicine in bariatric surgery prescription
Reviews in Endocrine and Metabolic Disorders (2023)
-
Comparison of Single Versus Double Anastomosis Bariatric Metabolic Surgery in Obesity: a Systematic Review and Meta-analysis
Obesity Surgery (2023)
-
Consistent gut bacterial and short-chain fatty acid signatures in hypoabsorptive bariatric surgeries correlate with metabolic benefits in rats
International Journal of Obesity (2022)