Abstract
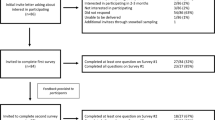
In Japan, most genetic testing for intractable diseases has been conducted in research laboratories in the past. However, since the Revised Medical Care Act came into effect on December 1, 2018, genetic testing in compliance with this act has become a major issue. To collect information on this topic, we conducted an online survey of members of the research groups for intractable diseases, which play a central role in medical care and research on intractable diseases with the support of the Ministry of Health, Labor and Welfare, five months after the enactment of the act. We separated the surveyed facilities into those that conducted genetic testing in their own laboratories (“testing facilities”) and those that outsourced genetic testing (“outsourcing facilities”). Ninety-five and 66 responses regarding genetic testing were obtained from the testing and outsourcing facilities, respectively. Genetic analysis was the most commonly conducted genetic testing method, accounting for 60% or more of the tests. At the testing facilities that conducted comprehensive analysis with a next-generation sequencer, the number of target diseases for genetic testing was observed to be higher. In these testing facilities, more than 70% were research laboratories. In contrast, at the outsourcing facilities, testing was outsourced to registered clinical laboratories in many cases or to research laboratories. The proportion of genetic testing covered by public medical insurance at the outsourcing facilities was two times higher than that at the testing facilities. The importance of quality control for genetic testing was generally well acknowledged, but there was apprehension regarding the increased cost and burden on staff of quality control assurance, and many testing facilities viewed genetic testing as difficult. The research groups could handle the examination and interpretation of the genetic testing results, and many groups gathered and registered patient information. Within the intractable disease medical support network, there was a relatively large number of collaborations, with studies supported by the Japan Agency for Medical Research and Development (AMED) and Initiative on Rare and Undiagnosed Diseases (IRUD) projects. There were many requests for genetic testing to be covered by public medical insurance. In the future, the implementation of genetic testing using a next-generation sequencer at clinical laboratories with guaranteed quality control and the development of a system for collaboration with research groups will be necessary.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
OMIM Gene Map Statistics https://omim.org/statistics/geneMap
Genetic Testing Registry (GTR) https://www.ncbi.nlm.nih.gov/gtr/
EuroGentest http://www.eurogentest.org
Medical Care Act (Act No. 205 of 1948) (enforced on December 1, 2018) https://www.mhlw.go.jp/content/10800000/000402678.pdf
Regulations on Medical Care Act Enforcement (Ordinance No. 50 of 1948 Ministry of Health and Welfare) (article 9-7, article 9-7-2, article 9-7-3, article 9-7-4, and article 9-8) (enforced on December 1, 2018) https://www.mhlw.go.jp/content/10800000/000402682.pdf
Mailing of Q&A Materials for Laboratory Tests Conducted at Medical Institutions and Clinical Laboratories https://www.mhlw.go.jp/content/10800000/qa_jimurenraku.pdf
Health and Labour Sciences Research Grant Research Project on Intractable Diseases Policy: Research Team for a Quality Control System for Laboratory Tests in the Field of Intractable Diseases (the Research Team) http://www.kentaikensa.jp/
Schreier J, Feeney R, Keeling P. Diagnosis reform and harmonization of Clinical laboratory testing. J Mol Diag. 2019;21:737–45.
Acknowledgements
The present study was funded by a Health and Labor Sciences Research Grant in Japan, Research Project on Intractable Diseases Policy “A Research Team for a Quality Control System for Laboratory Tests in the Field of Intractable Diseases (H30-Intractable Diseases-018)” (Principal Investigator: Eiji Nanba). We thank the following coinvestigators in the research team for their collaboration: Dr. Osamu Ohara (Kazusa DNA Research Institute), Dr. Hayato Miyachi (Tokai University), Dr. Tomohiro Nakayama (Nihon University), Dr. Tomoki Kosho (Shinshu University), Dr. Tadashi Kaname (National Center for Child Health and Development), Dr. Naoki Harada (Kyoto University), Mr. Masayoshi Tsutsumi (Japan Registerd Clinical Laboratories Association), Dr. Torayuki Okuyama (National Center for Child Health and Development), and Dr. Yu-ichi Goto (National Center of Neurology and Psychiatry).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adachi, K., Satou, K. & Nanba, E. Online questionnaire on genetic testing for intractable diseases in Japan: response to and issues associated with the revised medical care act. J Hum Genet 66, 1043–1051 (2021). https://doi.org/10.1038/s10038-021-00926-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00926-w
This article is cited by
-
Precision management of gene-based tests not covered by the National Health Insurance system in Japan: a questionnaire-based study
Journal of Human Genetics (2022)