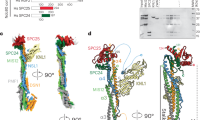
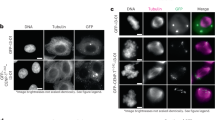
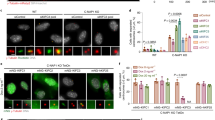
Abstract
Kinetochores are multicomponent assemblies that connect chromosomal centromeres to mitotic-spindle microtubules. The Ndc80 complex is an essential core element of kinetochores, conserved from yeast to humans. It is a rod-like assembly of four proteins— Ndc80p (HEC1 in humans), Nuf2p, Spc24p and Spc25p. We describe here the crystal structure of the most conserved region of HEC1, which lies at one end of the rod and near the N terminus of the polypeptide chain. It folds into a calponin-homology domain, resembling the microtubule-binding domain of the plus-end-associated protein EB1. We show that an Ndc80p-Nuf2p heterodimer binds microtubules in vitro. The less conserved, N-terminal segment of Ndc80p contributes to the interaction and may be a crucial regulatory element. We propose that the Ndc80 complex forms a direct link between kinetochore core components and spindle microtubules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Koshland, D.E., Mitchison, T.J. & Kirschner, M.W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature 331, 499–504 (1988).
Cleveland, D.W., Mao, Y. & Sullivan, K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421 (2003).
McAinsh, A.D., Tyell, J.D. & Sorger, P.K. Structure, function, and regulation of budding yeast kinetochores. Annu. Rev. Cell Dev. Biol. 19, 519–539 (2003).
Joglekar, A.P., Bouck, D.C., Molk, J.N., Bloom, K.S. & Salmon, E.D. Molecular architecture of a kinetochore-microtubule attachment site. Nat. Cell Biol. 8, 581–585 (2006).
Roos, U.P. Light and electron microscopy of rat kangaroo cells in mitosis. II. Kinetochore structure and function. Chromosoma 41, 195–220 (1973).
Chikashige, Y. et al. Composite motifs and repeat symmetry in S. pombe centromeres: direct analysis by integration of NotI restriction sites. Cell 57, 739–751 (1989).
Clarke, L. & Carbon, J. Isolation of the centromere-linked CDC10 gene by complementation in yeast. Proc. Natl. Acad. Sci. USA 77, 2173–2177 (1980).
Cottarel, G., Shero, J.H., Hieter, P. & Hegemann, J.H. A 125-base-pair CEN6 DNA fragment is sufficient for complete meiotic and mitotic centromere functions in Saccharomyces cerevisiae. Mol. Cell. Biol. 9, 3342–3349 (1989).
Peterson, J.B. & Ris, H. Electron-microscopic study of the spindle and chromosome movement in the yeast Saccharomyces cerevisiae. J. Cell Sci. 22, 219–242 (1976).
McDonald, K.L., O'Toole, E.T., Mastronarde, D.N. & McIntosh, J.R. Kinetochore microtubules in PTK cells. J. Cell Biol. 118, 369–383 (1992).
Winey, M. et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 129, 1601–1615 (1995).
Meraldi, P., McAinsh, A.D., Rheinbay, E. & Sorger, P.K. Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol. 7, R23 (2006).
Kline-Smith, S.L., Sandall, S. & Desai, A. Kinetochore-spindle microtubule interactions during mitosis. Curr. Opin. Cell Biol. 17, 35–46 (2005).
Deluca, J.G. et al. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell 16, 519–531 (2005).
He, X., Rines, D.R., Espelin, C.W. & Sorger, P.K. Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell 106, 195–206 (2001).
Janke, C., Ortiz, J., Tanaka, T.U., Lechner, J. & Schiebel, E. Four new subunits of the Dam1-Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J. 21, 181–193 (2002).
McCleland, M.L. et al. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101–114 (2003).
Li, Y. et al. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Genes Dev. 16, 183–197 (2002).
Tirnauer, J.S., Grego, S., Salmon, E.D. & Mitchison, T.J. EB1-microtubule interactions in Xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of rargeting to microtubules. Mol. Biol. Cell 13, 3614–3626 (2002).
Wei, R.R., Sorger, P.K. & Harrison, S.C. Molecular organization of the Ndc80 complex, an essential kinetochore component. Proc. Natl. Acad. Sci. USA 102, 5363–5367 (2005).
Gillett, E.S., Espelin, C.W. & Sorger, P.K. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 164, 535–546 (2004).
Ciferri, C. et al. Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J. Biol. Chem. 280, 29088–29095 (2005).
Wei, R.R. et al. Structure of a central component of the yeast dinetochore: rhe Spc24p/Spc25p globular domain. Structure 14, 1003–1009 (2006).
Hayashi, I. & Ikura, M. Crystal structure of the amino-terminal microtubule-binding domain of end-binding protein 1 (EB1). J. Biol. Chem. 278, 36430–36434 (2003).
Holm, L. & Sander, C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 233, 123–138 (1993).
Goldsmith, S.C. et al. The structure of an actin-crosslinking domain from human fimbrin. Nat. Struct. Biol. 4, 708–712 (1997).
Gimona, M., Djinovic-Carugo, K., Kranewitter, W.J. & Winder, S.J. Functional plasticity of CH domains. FEBS Lett. 513, 98–106 (2002).
Matsudaira, P. Modular organization of actin crosslinking proteins. Trends Biochem. Sci. 16, 87–92 (1991).
Klein, M.G. et al. Structure of the actin crosslinking core of fimbrin. Structure 12, 999–1013 (2004).
Borrego-Diaz, E. et al. Crystal structure of the actin-binding domain of [alpha]-actinin 1: Evaluating two competing actin-binding models. J. Struct. Biol. 155, 230–238 (2006).
Tirnauer, J.S. & Bierer, B.E. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J. Cell Biol. 149, 761–766 (2000).
Slep, K.C. et al. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 168, 587–598 (2005).
Tanaka, T.U. et al. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by atering kinetochore-spindle pole connections. Cell 108, 317–329 (2002).
Lampson, M.A., Renduchitala, K., Khodjakov, A. & Kapoor, T.M. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 6, 232–237 (2004).
Cheeseman, I.M. et al. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163–172 (2002).
Nousiainen, M., Sillje, H.H.W., Sauer, G., Nigg, E.A. & Korner, R. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA 103, 5391–5396 (2006).
DeLuca, J.G. et al. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127, 969–982 (2006).
Cheeseman, I.M. et al. The conserved KMN network constitutes the core microtubule- binding site of the kinetochore. Cell 127, 987–997 (2006).
Tanaka, K. et al. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature 434, 987–994 (2005).
Miranda, J.J., De Wulf, P., Sorger, P. & Harrison, S.C. The yeast DASH complex forms closed rings on microtubules. Nat. Struct. Mol. Biol. 12, 138–143 (2005).
Westermann, S. et al. Formation of a dynamic kinetochore-microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T.C. & Berendzen, J. Automated MAD and MIR structure solution. Acta Crystallograph. D Biol. Crystallogr. 55, 849–861 (1999).
Terwilliger, T. Maximum-likelihood density modification. Acta Crystallograph. D Biol. Crystallogr. 56, 965–972 (2000).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Roger, B., Al-Bassam, J., Dehmelt, L., Milligan, R.A. & Halpain, S. MAP2c, but not Tau, binds and bundles F-actin via its microtubule binding domain. Curr. Biol. 14, 363–371 (2004).
Honig, B. & Nicholls, A. Classical electrostatics in biology and chemistry. Science 268, 1144–1149 (1995).
Chenna, R. et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500 (2003).
Acknowledgements
The authors thank J.J. Miranda for help with the microtubule cosedimentation assay, D. Panne for assistance with X-ray data collection and E. Salmon, J. Deluca, N. Larsen, P.R. Ohi and V. Draviam for critique of the manuscript. We acknowledge the Advanced Light Source at Lawrence Berkeley National Laboratory. J.A.-B. is a recipient of the American Cancer Society postdoctoral fellowship. S.C.H. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
R.R.W. contributed to the design and execution of the structure determination of HEC1_CH, the microtubule cosedimentation experiments and manuscript preparation. J.A.-B. and R.R.W. contributed the negative-stain electron microscopy. S.C.H. guided the project and helped prepare the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Comparison of relative microtubule-binding affinity of Ndc80 and EB1. (PDF 68 kb)
Supplementary Fig. 2
Ndc80p N-terminal segment alone does not bind to microtubules. (PDF 11 kb)
Rights and permissions
About this article
Cite this article
Wei, R., Al-Bassam, J. & Harrison, S. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat Struct Mol Biol 14, 54–59 (2007). https://doi.org/10.1038/nsmb1186
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb1186
This article is cited by
-
Kinetochore component function in C. elegans oocytes revealed by 4D tracking of holocentric chromosomes
Nature Communications (2023)
-
Prc1-rich kinetochores are required for error-free acentrosomal spindle bipolarization during meiosis I in mouse oocytes
Nature Communications (2020)
-
Dynamics of kinetochore structure and its regulations during mitotic progression
Cellular and Molecular Life Sciences (2020)
-
Small molecules targeted to the microtubule–Hec1 interaction inhibit cancer cell growth through microtubule stabilization
Oncogene (2018)
-
Electrostatic forces drive poleward chromosome motions at kinetochores
Cell Division (2016)