Abstract
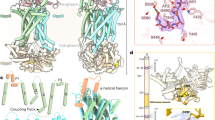
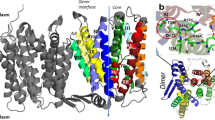
TamA is an Escherichia coli Omp85 protein involved in autotransporter biogenesis. It comprises a 16-stranded transmembrane β-barrel and three POTRA domains. The 2.3-Å crystal structure reveals that the TamA barrel is closed at the extracellular face by a conserved lid loop. The C-terminal β-strand of the barrel forms an unusual inward kink, which weakens the lateral barrel wall and creates a gate for substrate access to the lipid bilayer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Primary accessions
Protein Data Bank
Referenced accessions
NCBI Reference Sequence
Protein Data Bank
References
Chacinska, A., Koehler, C.M., Milenkovic, D., Lithgow, T. & Pfanner, N. Cell 138, 628–644 (2009).
Walther, D.M., Rapaport, D. & Tommassen, J. Cell. Mol. Life Sci. 66, 2789–2804 (2009).
Kim, S. et al. Science 317, 961–964 (2007).
Fan, E., Fiedler, S., Jacob-Dubuisson, F. & Müller, M. J. Biol. Chem. 287, 2591–2599 (2012).
Hagan, C.L., Silhavy, T.J. & Kahne, D. Annu. Rev. Biochem. 80, 189–210 (2011).
Wiedemann, N. et al. Nature 424, 565–571 (2003).
Jacob-Dubuisson, F., Guérin, J., Baelen, S. & Clantin, B. Res. Microbiol. 164, 583–595 (2013).
Clantin, B. et al. Science 317, 957–961 (2007).
Stegmeier, J.F., Glück, A., Sukumaran, S., Mäntele, W. & Andersen, C. Biol. Chem. 388, 37–46 (2007).
Selkrig, J. et al. Nat. Struct. Mol. Biol. 19, 506–510 (2012).
Vestweber, D., Brunner, J., Baker, A. & Schatz, G. Nature 341, 205–209 (1989).
Pusnik, M. et al. Curr. Biol. 21, 1738–1743 (2011).
Gatzeva-Topalova, P.Z., Warner, L.R., Pardi, A. & Sousa, M.C. Structure 18, 1492–1501 (2010).
Delattre, A.S. et al. Mol. Microbiol. 81, 99–112 (2011).
Delattre, A.S. et al. FEBS J. 277, 4755–4765 (2010).
Pavlova, O., Peterson, J.H., Ieva, R. & Bernstein, H.D. Proc. Natl. Acad. Sci. USA 110, E938–E947 (2013).
Guédin, S. et al. J. Biol. Chem. 275, 30202–30210 (2000).
van den Berg, B. J. Mol. Biol. 396, 627–633 (2010).
Bernstein, H.D. Trends Microbiol. 15, 441–447 (2007).
Saurí, A., Ten Hagen-Jongman, C.M., van Ulsen, P. & Luirink, J. J. Mol. Biol. 416, 335–345 (2012).
Noinaj, N. et al. Nature doi:10.1038/nature12521 (1 September 2013).
Betancor, L., Fernández, M.J., Weissman, K.J. & Leadlay, P.F. ChemBioChem 9, 2962–2966 (2008).
Kabsch, W. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Sheldrick, G.M. Acta Crystallogr. D Biol. Crystallogr. 66, 479–485 (2010).
McCoy, A.J. et al. J. Appl. Crystallogr. 40, 658–674 (2007).
Langer, G., Cohen, S.X., Lamzin, V.S. & Perrakis, A. Nat. Protoc. 3, 1171–1179 (2008).
Emsley, P. & Cowtan, K. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P.D. et al. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Sievers, F. et al. Mol. Syst. Biol. 7, 539 (2011).
Goujon, M. et al. Nucleic Acids Res. 38, W695–W699 (2010).
Pei, J. & Grishin, N.V. Bioinformatics 17, 700–712 (2001).
Acknowledgements
Crystallographic experiments were performed at PXIII (Swiss Light Source, Paul Scherrer Institute, Switzerland). We thank M. Wang and V. Olieric for support at the beamline, T. Schirmer for discussion and L. Betancor and P.F. Leadlay (University of Cambridge) for the pL1SL2 plasmid. This work was supported by the Swiss National Science Foundation (Grant PP00P3_128419 to S.H.) and the European Research Council (FP7 contract MOMP 281764 to S.H.). F.G. acknowledges a fellowship by the Werner-Siemens Foundation.
Author information
Authors and Affiliations
Contributions
S.H. and T.M. designed the study and guided the research experiments. F.G., F.Z., R.P.J. and B.M.B. carried out the experiments. All authors analyzed data. F.G., S.H. and T.M. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 1 (PDF 2325 kb)
Rights and permissions
About this article
Cite this article
Gruss, F., Zähringer, F., Jakob, R. et al. The structural basis of autotransporter translocation by TamA. Nat Struct Mol Biol 20, 1318–1320 (2013). https://doi.org/10.1038/nsmb.2689
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2689
This article is cited by
-
Surveying membrane landscapes: a new look at the bacterial cell surface
Nature Reviews Microbiology (2023)
-
Mechanisms of membrane protein crystallization in ‘bicelles’
Scientific Reports (2022)
-
Bacterial outer membrane proteins assemble via asymmetric interactions with the BamA β-barrel
Nature Communications (2019)
-
Membrane Protein Integration and Topogenesis at the ER
The Protein Journal (2019)
-
Molecular basis for the folding of β-helical autotransporter passenger domains
Nature Communications (2018)