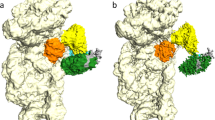
Abstract
Eukaryotic translation initiation factors (eIFs) 1A and 1 are central players in the complex process of start-codon recognition. To improve mechanistic understanding of this process, we determined the crystal structure of the 40S ribosomal subunit in complex with eIF1A and eIF1 from Tetrahymena thermophila at a resolution of 3.7 Å. It reveals the positions of the two factors on the 40S and the conformational changes that accompany their binding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hinnebusch, A.G. & Lorsch, J.R. Cold Spring Harb. Perspect. Biol. 4, a011544 (2012).
Pestova, T.V., Borukhov, S.I. & Hellen, C.U. Nature 394, 854–859 (1998).
Fekete, C.A. et al. EMBO J. 24, 3588–3601 (2005).
Nanda, J.S. et al. J. Mol. Biol. 394, 268–285 (2009).
Acker, M.G., Shin, B.S., Dever, T.E. & Lorsch, J.R. J. Biol. Chem. 281, 8469–8475 (2006).
Rabl, J., Leibundgut, M., Ataide, S.F., Haag, A. & Ban, N. Science 331, 730–736 (2011).
Battiste, J.L., Pestova, T.V., Hellen, C.U. & Wagner, G. Mol. Cell 5, 109–119 (2000).
Yu, Y. et al. Nucleic Acids Res. 37, 5167–5182 (2009).
Ben-Shem, A. et al. Science 334, 1524–1529 (2011).
Carter, A.P. et al. Science 291, 498–501 (2001).
Saini, A.K., Nanda, J.S., Lorsch, J.R. & Hinnebusch, A.G. Genes Dev. 24, 97–110 (2010).
Olsen, D.S. et al. EMBO J. 22, 193–204 (2003).
Marintchev, A., Kolupaeva, V.G., Pestova, T.V. & Wagner, G. Proc. Natl. Acad. Sci. USA 100, 1535–1540 (2003).
Ogle, J.M. et al. Science 292, 897–902 (2001).
Maag, D. & Lorsch, J.R. J. Mol. Biol. 330, 917–924 (2003).
Qin, D. & Fredrick, K. Mol. Microbiol. 71, 1239–1249 (2009).
Shin, B.S. et al. Nat. Struct. Mol. Biol. 18, 1227–1234 (2011).
Passmore, L.A. et al. Mol. Cell 26, 41–50 (2007).
Fekete, C.A. et al. EMBO J. 26, 1602–1614 (2007).
Voorhees, R.M., Weixlbaumer, A., Loakes, D., Kelley, A.C. & Ramakrishnan, V. Nat. Struct. Mol. Biol. 16, 528–533 (2009).
Kabsch, W. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Karplus, P.A. & Diederichs, K. Science 336, 1030–1033 (2012).
McCoy, A.J. et al. J. Appl. Crystallogr. 40, 658–674 (2007).
Adams, P.D. et al. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Jones, T.A., Zou, J.Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Laskowski, R.A., Rullmannn, J.A., MacArthur, M.W., Kaptein, R. & Thornton, J.M. J. Biomol. NMR 8, 477–486 (1996).
Baker, N.A., Sept, D., Joseph, S., Holst, M.J. & McCammon, J.A. Proc. Natl. Acad. Sci. USA 98, 10037–10041 (2001).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. J. Mol. Biol. 215, 403–410 (1990).
Larkin, M.A. et al. Bioinformatics 23, 2947–2948 (2007).
Waterhouse, A.M., Procter, J.B., Martin, D.M., Clamp, M. & Barton, G.J. Bioinformatics 25, 1189–1191 (2009).
Bond, C.S. & Schuttelkopf, A.W. Acta Crystallogr. D Biol. Crystallogr. 65, 510–512 (2009).
Klinge, S., Voigts-Hoffmann, F., Leibundgut, M. & Ban, N. Trends Biochem. Sci. 37, 189–198 (2012).
Acknowledgements
All data were collected at the Swiss Light Source (SLS, Paul Scherrer Institut, Villigen). We thank T. Tomizaki, M. Müller, V. Olieric, M. Wang, C. Pradervand, M. Marsh and A. Pauluhn for their outstanding support at the SLS; A. Haag for advice on cell growth, ribosome purification and eIF cloning and purification; V. Hozjan and F. Benning for experimental support; and D. Böhringer, B. Greber and A. Casañas for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (SNSF) (grant 31003A 144214 to N.B.), the National Center of Excellence in Research Structural Biology program of the SNSF (N.B.) and the European Community's Seventh Framework Programme (Project PF7 250071 to N.B.).
Author information
Authors and Affiliations
Contributions
J.R. cloned the initiation factors. M.W. and J.R. purified the initiation factors. M.W. crystallized the eIF1–eIF1A–40S complex. M.W. and M.L. solved the crystal structure. M.W., M.L., F.V.-H. and N.B. analyzed the data, interpreted the structure and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Tables 1–3 (PDF 10686 kb)
Rights and permissions
About this article
Cite this article
Weisser, M., Voigts-Hoffmann, F., Rabl, J. et al. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat Struct Mol Biol 20, 1015–1017 (2013). https://doi.org/10.1038/nsmb.2622
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2622
This article is cited by
-
The molecular basis of translation initiation and its regulation in eukaryotes
Nature Reviews Molecular Cell Biology (2024)
-
Mechanisms and regulation of protein synthesis in mitochondria
Nature Reviews Molecular Cell Biology (2021)
-
Cryo-EM study of an archaeal 30S initiation complex gives insights into evolution of translation initiation
Communications Biology (2020)
-
Translation complex profile sequencing to study the in vivo dynamics of mRNA–ribosome interactions during translation initiation, elongation and termination
Nature Protocols (2017)
-
Cryo-EM study of start codon selection during archaeal translation initiation
Nature Communications (2016)