Abstract
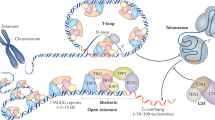
Telomeres are specialized DNA–protein complexes that stabilize chromosome ends, protecting them from nucleolytic degradation and illegitimate recombination. Telomeres form a heterochromatic structure that can suppress the transcription of adjacent genes. These structures might have additional roles in Trypanosoma brucei, as the major surface antigens of this parasite are expressed during its infectious stages from subtelomeric loci. We propose that the telomere protein complexes of trypanosomes and vertebrates are conserved and offer the hypothesis that growth and breakage of telomeric repeats has an important role in regulating parasite antigenic variation in trypanosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Barry, J. D. & McCulloch, R. Antigenic variation in trypanosomes: enhanced phenotypic variation in a eukaryotic parasite. Adv. Parasitol. 49, 1–70 (2001).
Navarro, M. & Gull, K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature 414, 759–763 (2001).
de Lange, T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 19, 2100–2110 (2005).
Smogorzewska, A. & de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 73, 177–208 (2004).
Tham, W. H. & Zakian, V. A. Transcriptional silencing at Saccharomyces telomeres: implications for other organisms. Oncogene 21, 512–521 (2002).
Chakhparonian, M. & Wellinger, R. J. Telomere maintenance and DNA replication: how closely are these two connected? Trends Genet. 19, 439–446 (2003).
Forstemann, K., Hoss, M. & Lingner, J. Telomerase-dependent repeat divergence at the 3′ends of yeast telomeres. Nucleic Acids Res. 28, 2690–2694 (2000).
Borst, P. & van Leeuwen, F. β-D-glucosyl-hydroxymethyluracil, a novel base in African trypanosomes and other Kinetoplastida. Mol. Biochem. Parasitol. 90, 1–8 (1997).
DiPaolo, C., Kieft, R., Cross, M. & Sabatini, R. Regulation of trypanosome DNA glycosylation by a SWI2/SNF2-like protein. Mol. Cell 17, 441–451 (2005).
van Leeuwen, F. et al. β-D-glucosyl-hydroxymethyluracil is a conserved DNA modification in kinetoplastid protozoans and is abundant in their telomeres. Proc. Natl Acad. Sci. USA 95, 2366–2371 (1998).
Horn, D. & Barry, J. D. The central roles of telomeres and subtelomeres in antigenic variation in African trypanosomes. Chromosome Res. 13, 525–533 (2005).
Horn, D., Spence, C. & Ingram, A. K. Telomere maintenance and length regulation in Trypanosoma brucei. EMBO J. 19, 2332–2339 (2000).
Glover, L. & Horn, D. Repression of polymerase I-mediated gene expression at Trypanosoma brucei telomeres. EMBO Rep. 7, 93–99 (2006).
Janzen, C. J., Lander, F., Dreesen, O. & Cross, G. A. M. Telomere length regulation and transcriptional silencing in KU80-deficient Trypanosoma brucei. Nucleic Acids Res. 32, 6575–6584 (2004).
Dreesen, O., Li, B. & Cross, G. A. M. Telomere structure and shortening in telomerase-deficient Trypanosoma brucei. Nucleic Acids Res. 33, 4536–4543 (2005).
Li, B., Espinal, A. & Cross, G. A. Trypanosome telomeres are protected by a homologue of mammalian TRF2. Mol. Cell. Biol. 25, 5011–5021 (2005).
Sfeir, A. J., Chai, W., Shay, J. W. & Wright, W. E. Telomere-end processing the terminal nucleotides of human chromosomes. Mol. Cell 18, 131–138 (2005).
Hockemeyer, D., Sfeir, A. J., Shay, J. W., Wright, W. E. & de Lange, T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. EMBO J. 24, 2667–2678 (2005).
Chiurillo, M. A. & Ramirez, J. L. Characterization of Leishmania major Friedlin telomeric terminus. Mem. Inst. Oswaldo Cruz, 97, 343–346 (2002).
de Lange, T. T-loops and the origin of telomeres. Nature Rev. Mol. Cell. Biol. 5, 323–329 (2004).
Munoz-Jordan, J. L., Cross, G. A. M., de Lange, T. & Griffith, J. D. t-Loops at trypanosome telomeres. EMBO J. 20, 579–588 (2001).
Nikitina, T. & Woodcock, C. L. Closed chromatin loops at the ends of chromosomes. J. Cell Biol. 166, 161–165 (2004).
Tomaska, L., Willcox, S., Slezakova, J., Nosek, J. & Griffith, J. D. Taz1 binding to a fission yeast model telomere: formation of telomeric loops and higher order structures. J. Biol. Chem., 279, 50764–50772 (2004).
Bernards, A., Michels, P. A., Lincke, C. R. & Borst, P. Growth of chromosome ends in multiplying trypanosomes. Nature 303, 592–597 (1983).
Pays, E., Laurent, M., Delinte, K., Van Meirvenne, N. & Steinert, M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 11, 8137–8147 (1983).
Dreesen, O. & Cross, G. A. M. Telomerase-independent stabilization of short telomeres in Trypanosoma brucei. Mol. Cell. Biol. 26, 4911–4919 (2006).
Kelleher, C., Teixeira, M. T., Forstemann, K. & Lingner, J. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem. Sci. 27, 572–579 (2002).
Huffman, K. E., Levene, S. D., Tesmer, V. M., Shay, J. W. & Wright, W. E. Telomere shortening is proportional to the size of the G-rich telomeric 3′-overhang. J. Biol. Chem. 275, 19719–19722 (2000).
Wright, W. E. & Shay, J. W. Historical claims and current interpretations of replicative aging. Nature Biotechnol. 20, 682–688 (2002).
Munoz, D. P. & Collins, K. Biochemical properties of Trypanosoma cruzi telomerase. Nucleic Acids Res. 32, 5214–5222 (2004).
Cano, M. I., Dungan, J. M., Agabian, N. & Blackburn, E. H. Telomerase in kinetoplastid parasitic protozoa. Proc. Natl Acad. Sci. USA 96, 3616–3621 (1999).
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003).
Counter, C. M. et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 11, 1921–1929 (1992).
Reddel, R. R. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 194, 155–162 (2003).
McEachern, M. J. & Haber, J. E. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75, 111–135 (2006).
Kyrion, G., Liu, K., Liu, C. & Lustig, A. J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 7, 1146–1159 (1993).
Halme, A., Bumgarner, S., Styles, C. & Fink, G. R. Genetic and epigenetic regulation of the FLO gene family generates cell-surface variation in yeast. Cell 116, 405–415 (2004).
Horn, D. & Cross, G. A. M. A developmentally regulated position effect at a telomeric locus in Trypanosoma brucei. Cell 83, 555–561 (1995).
Castano, I. et al. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol. Microbiol. 55, 1246–1258 (2005).
Duraisingh, M. T. et al. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell 121, 13–24 (2005).
Lowell, J. E. & Cross, G. A. M. A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J. Cell Sci. 117, 5937–5947 (2004).
Harper, L., Golubovskaya, I. & Cande, W. Z. A bouquet of chromosomes. J. Cell. Sci. 117, 4025–4032 (2004).
Dynek, J. N. & Smith, S. Resolution of sister telomere association is required for progression through mitosis. Science 304, 97–100 (2004).
de Lange, T. in Telomeres 2nd ed. (eds de Lange, T., Lundblad, V. and Blackburn, E. H.) 387–431 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 2006).
Maser, R. S. & DePinho, R. A. Telomeres and the DNA damage response: why the fox is guarding the henhouse. DNA Repair (Amst) 3, 979–988 (2004).
Fisher, T. S. & Zakian, V. A. Ku: a multifunctional protein involved in telomere maintenance. DNA Repair (Amst) 4, 1215–1226 (2005).
Stellwagen, A. E., Haimberger, Z. W., Veatch, J. R. & Gottschling, D. E. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17, 2384–2395 (2003).
Peterson, S. E. et al. The function of a stem-loop in telomerase RNA is linked to the DNA repair protein Ku. Nature Genet. 27, 64–67 (2001).
Robinson, N. P., McCulloch, R., Conway, C., Browitt, A. & Barry, J. D. Inactivation of Mre11 does not affect VSG gene duplication mediated by homologous recombination in Trypanosoma brucei. J. Biol. Chem. 277, 26185–26193 (2002).
Tan, K. S., Leal, S. T. & Cross, G. A. M. Trypanosoma brucei MRE11 is non-essential but influences growth, homologous recombination and DNA double-strand break repair. Mol. Biochem. Parasitol. 125, 11–21 (2002).
Li, B., Oestreich, S. & de Lange, T. Identification of human Rap1: implications for telomere evolution. Cell 101, 471–483 (2000).
Henderson, E. in Telomeres 1st Edn (eds Blackburn, E. H. and Greider, C.) 11–34 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1995).
Kanoh, J. & Ishikawa, F. Composition and conservation of the telomeric complex. Cell. Mol. Life Sci. 60, 2295–2302 (2003).
Vanhamme, L. et al. Differential RNA elongation controls the variant surface glycoprotein gene expression sites of Trypanosoma brucei. Mol. Microbiol. 36, 328–340 (2000).
Wickstead, B., Ersfeld, K. & Gull, K. The small chromosomes of Trypanosoma brucei involved in antigenic variation are constructed around repetitive palindromes. Genome Res. 14, 1014–1024 (2004).
Borst, P. & Ulbert, S. Control of VSG gene expression sites. Mol. Biochem. Parasitol. 114, 17–27 (2001).
Morrison, L. J., Majiwa, P., Read, A. F. & Barry, J. D. Probabilistic order in antigenic variation of Trypanosoma brucei. Int. J. Parasitol. 35, 961–972 (2005).
Rudenko, G., McCulloch, R., Dirks-Mulder, A. & Borst, P. Telomere exchange can be an important mechanism of variant surface glycoprotein gene switching in Trypanosoma brucei. Mol. Biochem. Parasitol. 80, 65–75 (1996).
Myler, P. J., Allison, J., Agabian, N. & Stuart, K. Antigenic variation in African trypanosomes by gene replacement or activation of alternate telomeres. Cell, 39, 203–211 (1984).
Horn, D. & Cross, G. A. M. Analysis of Trypanosoma brucei VSG expression site switching in vitro. Mol. Biochem. Parasitol. 84, 189–201 (1997).
Conway, C. et al. Ku is important for telomere maintenance, but not for differential expression of telomeric VSG genes, in African trypanosomes. J. Biol. Chem. 277, 21269–21277 (2002).
McCulloch, R. & Barry, J. D. A role for RAD51 and homologous recombination in Trypanosoma brucei antigenic variation. Genes Dev. 13, 2875–2888 (1999).
Barry, J. D. The relative significance of mechanisms of antigenic variation in African trypanosomes. Parasitol. Today 13, 212–218 (1997).
Robinson, N. P., Burman, N., Melville, S. E. & Barry, J. D. Predominance of duplicative VSG gene conversion in antigenic variation in African trypanosomes. Mol. Cell. Biol. 19, 5839–5846 (1999).
Turner, C. M. & Barry, J. D. High frequency of antigenic variation in Trypanosoma brucei rhodesiense infections. Parasitology 99, 67–75 (1989).
Barry, J. D., Ginger, M. L., Burton, P. & McCulloch, R. Why are parasite contingency genes often associated with telomeres? Int. J. Parasitol. 33, 29–45 (2003).
Freitas-Junior, L. H. et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407, 1018–1022 (2000).
Pays, E. et al. The trypanolytic factor of human serum. Nature Reviews Microbiol. 4, 477–486 (2006)
Acknowledgements
We are grateful to Titia de Lange, Joachim Lingner, Ed Louis and the members of the Cross laboratory for excellent advice and discussions. Our work on telomeres was supported by the National Institutes of Health. We apologize to authors whose original work could not always be cited due to length restrictions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Dreesen, O., Li, B. & Cross, G. Telomere structure and function in trypanosomes: a proposal. Nat Rev Microbiol 5, 70–75 (2007). https://doi.org/10.1038/nrmicro1577
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1577
This article is cited by
-
Decoding the impact of nuclear organization on antigenic variation in parasites
Nature Microbiology (2023)
-
Does DNA replication direct locus-specific recombination during host immune evasion by antigenic variation in the African trypanosome?
Current Genetics (2017)
-
TRF2-RAP1 is required to protect telomeres from engaging in homologous recombination-mediated deletions and fusions
Nature Communications (2016)
-
Genome evolution in filamentous plant pathogens: why bigger can be better
Nature Reviews Microbiology (2012)
-
A yeast-endonuclease-generated DNA break induces antigenic switching in Trypanosoma brucei
Nature (2009)