Key Points
-
Multi-partner endosymbioses have evolved independently in several lineages of the plant sap-feeding insects of the order Hemiptera. Many of these endosymbionts have much-reduced genomes as a result of relaxed selection and genomic decay.
-
In multi-partner endosymbioses, the production of essential amino acids is often partitioned between the different endosymbionts, such that each endosymbiont mediates the biosynthesis of a subset of essential amino acids or a subset of the reactions in the biosynthetic pathway of a single essential amino acid.
-
The essential amino acid biosynthetic pathways that are partitioned between primary endosymbionts and more recently acquired endosymbionts are energetically costly, which suggests that the capacity for energy production may be limiting in primary endosymbionts, possibly as a result of genomic decay.
-
Rather than partition nutrient biosynthesis, the benefit to the host of some multi-partner endosymbioses relates to the acquisition of additional functions. In these examples, one endosymbiont specializes in providing a nutritional benefit, whereas the other endosymbiont provides a non-nutritional benefit, such as protection from natural enemies.
-
The phylogenetic distribution of the various endosymbiont taxa that colonize Hemiptera indicates that multi-partner endosymbioses have generally evolved through the sequential acquisition of different microorganisms, together with the occasional replacement of one or more endosymbionts by different taxa.
-
The absence of endosymbionts in, for example, some insect pollinators and vertebrates, including humans, may provide an opportunity to identify symbiosis-related molecular functions that can be targeted as novel pest control strategies.
Abstract
Various animals are associated with specific endosymbiotic microorganisms that provide the host with essential nutrients or confer protection against natural enemies. Genomic analyses of the many endosymbioses that are found in plant sap-feeding hemipteran insects have revealed independent acquisitions — and occasional replacements — of endosymbionts, such that many of these endosymbioses involve two or more microbial partners. In this Review, I discuss how partitioning of the genetic capacity for metabolic function between different endosymbionts has sustained nutritional function in multi-partner endosymbioses, and how the phenotypic traits of these endosymbionts can be shaped by co-evolutionary interactions with both co-occurring microbial taxa and the host, which often operate over long evolutionary timescales.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236 (2013).
The Human Microbiome Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Sender, R., Fuchs, S. & Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164, 337–340 (2016).
Lloyd-Price, J., Abu-Ali, G. & Huttenhower, C. The healthy human microbiome. Genome Med. 8, 51 (2016).
Douglas, A. E. The Symbiotic Habit (Princeton Univ. Press, 2010).
Douglas, A. E. Multiorganismal insects: diversity and function of resident microorganisms. Annu. Rev. Entomol. 60, 17–34 (2015).
Kerney, R. et al. Intracellular invasion of green algae in a salamander host. Proc. Natl Acad. Sci. USA 108, 6497–6502 (2011).
Moulder, J. W. The cell as an extreme environment. Proc. R. Soc. Lond. B Biol. Sci. 204, 199–210 (1979).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Ray, K., Marteyn, B., Sansonetti, P. J. & Tang, C. M. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 7, 333–340 (2009).
McCutcheon, J. P. & Moran, N. A. Extreme genome reduction in symbiotic bacteria. Nat. Rev. Microbiol. 10, 13–26 (2012).
Buchner, P. Endosymbioses of Animals with Plant Microorganisms (John Wiley and Sons, 1965).
Nakabachi, A. et al. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 23, 1478–1484 (2013). This study demonstrates that a bacterium that can synthesize a polyketide with predicted defensive function has evolved into an endosymbiont that has a very small genome and is absolutely dependent on its insect host, complementing the nutritional function of the primary symbiont.
Bennett, G. M. & Moran, N. A. Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a phloem-feeding insect. Genome Biol. Evol. 5, 1675–1688 (2013).
Toenshoff, E. R., Gruber, D. & Horn, M. Co-evolution and symbiont replacement shaped the symbiosis between adelgids (Hemiptera: Adelgidae) and their bacterial symbionts. Environ. Microbiol. 14, 1284–1295 (2012).
Toenshoff, E. R., Szabo, G., Gruber, D. & Horn, M. The pine bark adelgid, Pineus strobi, contains two novel bacteriocyte-associated gammaproteobacterial symbionts. Appl. Environ. Microbiol. 80, 878–885 (2014).
Gottlieb, Y. et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl. Environ. Microbiol. 72, 3646–3652 (2006).
McCutcheon, J. P. & von Dohlen, C. D. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr. Biol. 21, 1366–1372 (2011). The article provides the first description of a nested endosymbiosis, in which the genomes of the two endosymbionts encode different genes of certain EAA biosynthetic pathways.
Koga, R., Nikoh, N., Matsuura, Y., Meng, X. Y. & Fukatsu, T. Mealybugs with distinct endosymbiotic systems living on the same host plant. FEMS Microbiol. Ecol. 83, 93–100 (2013).
Husnik, F. & McCutcheon, J. P. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc. Natl Acad. Sci. USA 113, E5416–E5424 (2016).
Watanabe, K., Yukuhiro, F., Matsuura, Y., Fukatsu, T. & Noda, H. Intrasperm vertical symbiont transmission. Proc. Natl Acad. Sci. USA 111, 7433–7437 (2014).
De Vooght, L., Caljon, G., Van Hees, J. & Van Den Abbeele, J. Paternal transmission of a secondary symbiont during mating in the viviparous tsetse fly. Mol. Biol. Evol. 32, 1977–1980 (2015).
Oliver, T. A., Smith, A. H. & Russell, J. A. Defensive symbiosis in the real world — advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct. Ecol. 28, 341–355 (2014).
Moran, N. A., Russell, J. A., Koga, R. & Fukatsu, T. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71, 3302–3310 (2005).
Skaljac, M., Zanic, K., Ban, S. G., Kontsedalov, S. & Ghanim, M. Co-infection and localization of secondary symbionts in two whitefly species. BMC Microbiol. 10, 142 (2010).
Caspi-Fluger, A. et al. Rickettsia 'in' and 'out': two different localization patterns of a bacterial symbiont in the same insect species. PLoS ONE 6, e21096 (2011).
Koga, R., Tsuchida, T. & Fukatsu, T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. Biol. Sci. 270, 2543–2550 (2003).
Darby, A. C., Chandler, S. M., Welburn, S. C. & Douglas, A. E. Aphid-symbiotic bacteria cultured in insect cell lines. Appl. Environ. Microbiol. 71, 4833–4839 (2005).
Oliver, K. M., Moran, N. A. & Hunter, M. S. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. Biol. Sci. 273, 1273–1280 (2006).
Chandler, S. M., Wilkinson, T. L. & Douglas, A. E. Impact of plant nutrients on the relationship between a herbivorous insect and its symbiotic bacteria. Proc. Biol. Sci. 275, 565–570 (2008).
Oliver, K. M., Degnan, P. H., Burke, G. R. & Moran, N. A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55, 247–266 (2010).
Caspi-Fluger, A. et al. Horizontal transmission of the insect symbiont Rickettsia is plant-mediated. Proc. Biol. Sci. 279, 1791–1796 (2012). A very thorough study of horizontal transmission of a secondary symbiont that provides empirical evidence for one route contributing to the lack of congruence between the phylogenies of aphids and their secondary symbionts.
Oliver, K. M., Campos, J., Moran, N. A. & Hunter, M. S. Population dynamics of defensive symbionts in aphids. Proc. Biol. Sci. 275, 293–299 (2008).
Douglas, A. E., François, C. L. M. J. & Minto, L. B. Facultative 'secondary' bacterial symbionts and the nutrition of the pea aphid, Acyrthosiphon pisum. Physiol. Entomol. 31, 262–269 (2006).
Rothacher, L., Ferrer-Suay, M. & Vorburger, C. Bacterial endosymbionts protect aphids in the field and alter parasitoid community composition. Ecology 97, 1712–1723 (2016). An important study that demonstrates the significance of secondary symbionts to protection against natural enemies under field conditions, which complements many laboratory studies.
Hansen, A. K. & Moran, N. A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 23, 1473–1496 (2014).
Gerardo, N. M. & Parker, B. J. Mechanisms of symbiont-conferred protection against natural enemies: an ecological and evolutionary framework. Curr. Opin. Insect Sci. 4, 8–14 (2014).
Oliver, K. M., Degnan, P. H., Hunter, M. S. & Moran, N. A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325, 992–994 (2009).
Laughton, A. M., Garcia, J. R. & Gerardo, N. M. Condition-dependent alteration of cellular immunity by secondary symbionts in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 86, 17–24 (2016).
Caragata, E. P. et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9, e1003459 (2013).
Hufbauer, R. A. & Via, S. Evolution of an aphid–parasitoid interaction: variation in resistance to parasitism among aphid populations specialized on different plants. Evolution 53, 1435–1445 (1999).
Burke, G., Fiehn, O. & Moran, N. Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 4, 242–252 (2010).
Dunbar, H. E., Wilson, A. C., Ferguson, N. R. & Moran, N. A. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5, e96 (2007).
Ramsey, J. S. et al. Metabolic interplay between the Asian citrus psyllid and its Profftella symbiont: an Achilles' heel of the citrus greening insect vector. PLoS ONE 10, e0140826 (2015).
Sloan, D. B. & Moran, N. A. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol. Biol. Evol. 29, 3781–3792 (2012). This study provides a clear-cut demonstration of how the acquisition of different secondary symbionts in various psyllid lineages has led to the evolution of different patterns of partitioning of EAA biosynthesis reactions, which highlights ongoing evolutionary changes in multi-partner endosymbioses.
McCutcheon, J. P., McDonald, B. R. & Moran, N. A. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc. Natl Acad. Sci. USA 106, 15394–15399 (2009).
McCutcheon, J. P. & Moran, N. A. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol. Evol. 2, 708–718 (2010).
Lamelas, A., Gosalbes, M. J., Moya, A. & Latorre, A. New clues about the evolutionary history of metabolic losses in bacterial endosymbionts, provided by the genome of Buchnera aphidicola from the aphid Cinara tujafilina. Appl. Environ. Microbiol. 77, 4446–4454 (2011).
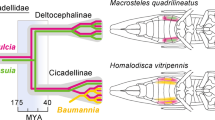
Van Leuven, J. T., Meister, R. C., Simon, C. & McCutcheon, J. P. Sympatric speciation in a bacterial endosymbiont results in two genomes with the functionality of one. Cell 158, 1270–1280 (2014). The study provides the first report of within-lineage partitioning of function in endosymbiotic bacteria, which was identified in the companion symbiont ' Ca . Hodgkinia cicadicola' in a cicada species.
Campbell, M. A. et al. Genome expansion via lineage splitting and genome reduction in the cicada endosymbiont Hodgkinia. Proc. Natl Acad. Sci. USA 112, 10192–10199 (2015).
Husnik, F. et al. Horizontal gene transfer from diverse bacteria to an insect genome enables a tripartite nested mealybug symbiosis. Cell 153, 1567–1578 (2013).
Matsuura, Y. et al. Huge symbiotic organs in giant scale insects of the genus Drosicha (Coccoidea: Monophlebidae) harbor flavobacterial and enterobacterial endosymbionts. Zoolog. Sci. 26, 448–456 (2009).
Dhami, M. K., Turner, A. P., Deines, P., Beggs, J. R. & Taylor, M. W. Ultrastructural and molecular characterization of a bacterial symbiosis in the ecologically important scale insect family Coelostomidiidae. FEMS Microbiol. Ecol. 81, 537–546 (2012).
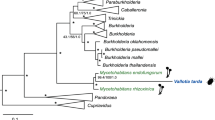
Koga, R., Bennett, G. M., Cryan, J. R. & Moran, N. A. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 15, 2073–2081 (2013).
Sloan, D. B. & Moran, N. A. The evolution of genomic instability in the obligate endosymbionts of whiteflies. Genome Biol. Evol. 5, 783–793 (2013).
Wernegreen, J. J. Mutualism meltdown in insects: bacteria constrain thermal adaptation. Curr. Opin. Microbiol. 15, 255–262 (2012).
Bennett, G. M. & Moran, N. A. Heritable symbiosis: The advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA 112, 10169–10176 (2015).
Gomez-Valero, L. et al. Coexistence of Wolbachia with Buchnera aphidicola and a secondary symbiont in the aphid Cinara cedri. J. Bacteriol. 186, 6626–6633 (2004).
Douglas, A. E. Molecular dissection of nutrient exchange at the insect–microbial interface. Curr. Opin. Insect Sci. 4, 23–28 (2014).
Douglas, A. E. Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol. 25, 338–342 (2007).
Mitri, S. & Foster, K. R. The genotypic view of social interactions in microbial communities. Annu. Rev. Genet. 47, 247–273 (2013).
West, S. A., Diggle, S. P., Buckling, A., Gardner, A. & Griffin, A. S. The social lives of microbes. Annu. Rev. Ecol. Evol. Syst. 38, 53–77 (2007).
Rakoff-Nahoum, S., Foster, K. R. & Comstock, L. E. The evolution of cooperation within the gut microbiota. Nature 533, 255–259 (2016).
Cryan, J. R. & Urban, J. M. Higher-level phylogeny of the insect order Hemiptera: is Auchenorrhyncha really paraphyletic? Syst. Entomol. 37, 7–21 (2012).
von Dohlen, C. D. & Moran, N. A. Molecular phylogeny of the Homoptera: a paraphyletic taxon. J. Mol. Evol. 41, 211–223 (1995).
Bourgoin, T. & Campbell, B. C. Inferring a phylogeny for Hemiptera: falling into the 'autapomorphic trap'. Denisia 4, 67–82 (2002).
Kikuchi, Y., Meng, X. Y. & Fukatsu, T. Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl. Environ. Microbiol. 71, 4035–4043 (2005).
Nikoh, N., Hosokawa, T., Oshima, K., Hattori, M. & Fukatsu, T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 3, 702–714 (2011).
Matsuura, Y. et al. Bacterial symbionts of a devastating coffee plant pest, the stinkbug Antestiopsis thunbergii (Hemiptera: Pentatomidae). Appl. Environ. Microbiol. 80, 3769–3775 (2014).
Nakabachi, A. & Ishikawa, H. Provision of riboflavin to the host aphid, Acyrthosiphon pisum, by endosymbiotic bacteria, Buchnera. J. Insect Physiol. 45, 1–6 (1999).
Fan, H. W. et al. Genomic analysis of an ascomycete fungus from the rice planthopper reveals how it adapts to an endosymbiotic lifestyle. Genome Biol. Evol. 7, 2623–2634 (2015).
Noda, H. & Koizumi, Y. Sterol biosynthesis by symbiotes: cytochrome P450 sterol C-22 desaturase genes from yeast-like symbiotes of rice planthoppers and anobiid beetles. Insect Biochem. Mol. Biol. 33, 649–658 (2003).
Moran, N. A. Accelerated evolution and Muller's rachet in endosymbiotic bacteria. Proc. Natl Acad. Sci. USA 93, 2873–2878 (1996).
Fares, M. A., Moya, A. & Barrio, E. GroEL and the maintenance of bacterial endosymbiosis. Trends Genet. 20, 413–416 (2004).
Price, D. R. et al. Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts. Proc. Natl Acad. Sci. USA 111, 320–325 (2014).
Duncan, R. P., Feng, H., Nguyen, D. M. & Wilson, A. C. Gene family expansions in aphids maintained by endosymbiotic and nonsymbiotic traits. Genome Biol. Evol. 8, 753–764 (2016).
Cocucci, E. & Meldolesi, J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 25, 364–372 (2015).
Pande, S. et al. Metabolic cross-feeding via intercellular nanotubes among bacteria. Nat. Commun. 6, 6238 (2015).
Russell, C. W., Bouvaine, S., Newell, P. D. & Douglas, A. E. Shared metabolic pathways in a coevolved insect–bacterial symbiosis. Appl. Environ. Microbiol. 79, 6117–6123 (2013).
Kaleta, C., Schauble, S., Rinas, U. & Schuster, S. Metabolic costs of amino acid and protein production in Escherichia coli. Biotechnol. J. 8, 1105–1114 (2013).
Poliakov, A. et al. Large-scale label-free quantitative proteomics of the pea aphid–Buchnera symbiosis. Mol. Cell. Proteomics 10, M110 007039 (2011).
Luan, J. B. et al. Metabolic coevolution in the bacterial symbiosis of whiteflies and related plant sap-feeding insects. Genome Biol. Evol. 7, 2635–2647 (2015).
Acknowledgements
Work in the author's laboratory is financially supported by the US National Science Foundation (NSF; grant IOS-1354743) and the Sarkaria Institute of Insect Physiology and Toxicology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Multi-partner symbioses in Hemipteran insects (PDF 173 kb)
Glossary
- Endosymbionts
-
Microorganisms that are localized to the internal organs, often within cells, of animals.
- Essential amino acids
-
(EAAs). Ten of the 20 amino acids that constitute proteins, but which cannot be synthesized by animals (some animals, but not hemipteran insects, have limited capacity to synthesize one essential amino acid, arginine).
- Stylet
-
Mouthparts of hemipteran insects, in which the mandibles and maxillae are modified to pierce into plant or animal tissues and take up liquid food.
- Primary symbionts
-
Endosymbionts that are required by an insect host and that are invariably vertically transmitted to the insect offspring.
- Bacteriocyte
-
A specialized insect cell that houses and maintains intracellular endosymbionts.
- Transovarial transmission
-
Vertical transmission of endosymbiotic microorganisms by transfer to the offspring insect through the ovaries of the reproductive female.
- Companion symbionts
-
Endosymbionts that co-occur with the primary symbiont 'Candidatus Sulcia muelleri' in the suborder Auchenorrhyncha. Companion symbionts are generally required by the insect and are vertically transmitted (similarly to primary symbionts). The identity of the companion symbionts varies among different auchenorrhynchan lineages.
- Secondary symbionts
-
Endosymbionts that co-occur with the primary symbiont in the suborder Sternorrhyncha. Some secondary symbionts are required by the insect, but many are facultative for the insect and show patterns of mixed vertical and horizontal transmission.
- Syncytium
-
A cell that contains several nuclei that are generated either through rounds of nuclear division without cytokinesis or through the fusion of multiple cells.
- Haemolymph
-
A blood-like fluid in insects.
- Nested endosymbiosis
-
Symbiosis in which the cells of one endosymbiont are localized within a cell of a second endosymbiont.
- Parasitoid
-
An insect that develops within a single insect host of a different species, which it kills before emerging as an adult.
- Encapsulation
-
An innate immune response of an insect by which a parasite is enclosed within many layers of haemocytes and killed, often by the cytotoxic products of the haemocytes.
- Epistatic interactions
-
Interactions between genes: the phenotypic effect of one gene is influenced by another gene.
- Polyketide
-
A group of complex aromatic secondary metabolites, which includes macrolides, polyenes and tetracyclines.
- Genomic decay
-
The accumulation of deleterious mutations and gene loss by genetic drift in organisms that have a small effective population size.
Rights and permissions
About this article
Cite this article
Douglas, A. How multi-partner endosymbioses function. Nat Rev Microbiol 14, 731–743 (2016). https://doi.org/10.1038/nrmicro.2016.151
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.151
This article is cited by
-
Frankia-actinorhizal symbiosis: a non-chemical biological assemblage for enhanced plant growth, nodulation and reclamation of degraded soils
Symbiosis (2024)
-
Nutrient supplementation by genome-eroded Burkholderia symbionts of scale insects
The ISME Journal (2023)
-
Vertical transmission of cellulolytic protists in termites is imperfect, but sufficient, due to biparental transmission
Symbiosis (2023)
-
Influence of factitious hosts on the morphometry and diversity of endosymbionts of the egg parasitoid Telenomus remus: insights for applied biological control
Phytoparasitica (2023)
-
Microbiome comparison of Dermanyssus gallinae populations from different farm rearing systems and the presence of common endosymbiotic bacteria at developmental stages
Parasitology Research (2023)