Key Points
-
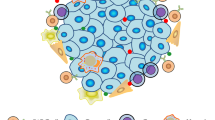
Despite having the power to protect us against infectious diseases, the immune system that Mother Nature provides ultimately fails when it comes to cancer. The same immune-system checks and balances that guard against autoimmunity lead to fundamental shortfalls and deficiencies in the antitumour capabilities of the endogenous immune system.
-
Our understanding of the molecular events that are important in the recognition of microorganisms by immune cells, and the downstream signalling processes that are important in mediating a response to these microbial threats, has led to the design of genes that can enable us to correct deficiencies in the anticancer immune response.
-
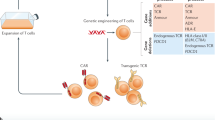
The endogenous immune system often lacks effective tumour-specific T cells, but specificity can be provided by genetic modification of 'ordinary' T cells with genes that encode cell-surface receptors that allow recognition of tumour cells. Examples of such receptors are T-cell receptors from rare responding patients or chimeric receptors that incorporate tumour-specific monoclonal antibodies.
-
It is possible to combine, within a single chimeric-receptor gene, molecular domains from several signal-transducing molecules to produce a 'super receptor' that endows T cells with increased antitumour activity.
-
When engaged in the struggle with tumour cells, natural T cells quickly 'tire', leading to their exhaustion or death. Various genetic strategies can be used to extend the lifespan of tumour-reactive T cells, with the possibility of producing the ultimate 'immortal' tumour-fighting cellular warriors.
-
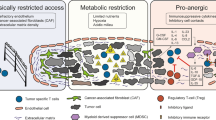
Tumours might lack appropriate molecular signals that are important for attracting endogenous tumour-specific T cells. Genetic modification can be used to guide these T cells and enable them to home to, and penetrate, tumour tissue.
-
Tumours can produce inhibitory factors that can dampen the immune response, but T cells can be transduced with genes that endow them with resistance to such factors, thereby allowing them to realize their full antitumour potential.
-
With further improvements in the technology and the safety of genetic-modification strategies, it might soon be possible to transduce ordinary T cells with multiple genes to endow them with a coordinated genetic programme that gives them extraordinary activity against cancer.
Abstract
Immunotherapy is receiving much attention as a means of treating cancer, but complete, durable responses remain rare for most malignancies. The natural immune system seems to have limitations and deficiencies that might affect its ability to control malignant disease. An alternative to relying on endogenous components in the immune repertoire is to generate lymphocytes with abilities that are greater than those of natural T cells, through genetic modification to produce 'supernatural' T cells. This Review describes how such T cells can circumvent many of the barriers that are inherent in the tumour microenvironment while optimizing T-cell specificity, activation, homing and antitumour function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Smyth, M. J., Godfrey, D. I. & Trapani, J. A. A fresh look at tumor immunosurveillance and immunotherapy. Nature Immunol. 2, 293–299 (2001).
Rosenberg, S. A., Fox, E. & Churchill, W. H. Spontaneous regression of hepatic metastases from gastric carcinoma. Cancer 29, 472–474 (1972).
Cole, W. H. Relationship of causative factors in spontaneous regression of cancer to immunologic factors possibly effective in cancer. J. Surg. Oncol. 8, 391–411 (1976).
Rosenberg, S. A. Progress in human tumour immunology and immunotherapy. Nature 411, 380–384 (2001).
Interferon α versus chemotherapy for chronic myeloid leukemia: a meta-analysis of seven randomized trials: Chronic Myeloid Leukemia Trialists' Collaborative Group. J. Natl Cancer Inst. 89, 1616–1620 (1997).
Grillo-Lopez, A. J., Hedrick, E., Rashford, M. & Benyunes, M. Rituximab: ongoing and future clinical development. Semin. Oncol. 29, 105–112 (2002).
Heslop, H. E. et al. Long-term restoration of immunity against Epstein–Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nature Med. 2, 551–555 (1996).
Walter, E. A. et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333, 1038–1044 (1995).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Rosenberg, S. A. et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med. 4, 321–327 (1998).
Khong, H. T. & Restifo, N. P. Natural selection of tumor variants in the generation of 'tumor escape' phenotypes. Nature Immunol. 3, 999–1005 (2002).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Zhao, Y. et al. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J. Immunol. 174, 4415–4423 (2005).
Kessels, H. W., Wolkers, M. C., van den Boom, M. D., van der Valk, M. A. & Schumacher, T. N. Immunotherapy through TCR gene transfer. Nature Immunol. 2, 957–961 (2001).
Stanislawski, T. et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nature Immunol. 2, 962–970 (2001).
Kuball, J. et al. Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity 22, 117–129 (2005).
Willemsen, R. A. et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 7, 1369–1377 (2000).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin–T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989). This is the original report describing the concept of redirecting T-cell function by genetic modification with chimeric receptors composed of antibody determinants linked to T-cell signalling components. In this study, a mouse T-cell hybridoma line was engineered to react with the hapten trinitrophenol conjugated to tumour cells.
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Hwu, P. et al. Lysis of ovarian cancer cells by human lymphocytes redirected with a chimeric gene composed of an antibody variable region and the Fc receptor γ chain. J. Exp. Med. 178, 361–366 (1993).
Altenschmidt, U., Klundt, E. & Groner, B. Adoptive transfer of in vitro-targeted, activated T lymphocytes results in total tumor regression. J. Immunol. 159, 5509–5515 (1997).
Hwu, P. et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res. 55, 3369–3373 (1995). This study shows the potential of genetically redirected T cells to influence tumour growth in mice. A gene that encoded a chimeric antibody–Fcγ receptor enabled adoptively transferred mouse T cells to inhibit early intraperitoneal or lung metastases.
Pinthus, J. H. et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J. Clin. Invest. 114, 1774–1781 (2004).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Med. 9, 279–286 (2003).
Mezzanzanica, D. et al. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 5, 401–407 (1998).
Rossig, C., Bollard, C. M., Nuchtern, J. G., Rooney, C. M. & Brenner, M. K. Epstein–Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood 99, 2009–2016 (2002).
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Andersen, P. S. et al. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc. Natl Acad. Sci. USA 93, 1820–1824 (1996).
Hombach, A. et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 6, 300–304 (1999).
Patel, S. D. et al. T-cell killing of heterogenous tumor or viral targets with bispecific chimeric immune receptors. Cancer Gene Ther. 7, 1127–1134 (2000).
Yee, C. et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J. Exp. Med. 192, 1637–1644 (2000).
Overwijk, W. W. et al. Vaccination with a recombinant vaccinia virus encoding a 'self' antigen induces autoimmune vitiligo and tumor cell destruction in mice: requirement for CD4+ T lymphocytes. Proc. Natl Acad. Sci. USA 96, 2982–2987 (1999).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Lamers, C. H. et al. Adoptive immuno-gene therapy of cancer with single chain antibody [scFv(Ig)] gene modified T lymphocytes. J. Biol. Regul. Homeost. Agents 18, 134–140 (2004).
Chen, W. et al. Reversal in the immunodominance hierarchy in secondary CD8+ T cell responses to influenza A virus: roles for cross-presentation and lysis-independent immunodomination. J. Immunol. 173, 5021–5027 (2004).
Lee, P. P. et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature Med. 5, 677–685 (1999).
Haynes, N. M. et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-ζ vs FcεRI-γ. J. Immunol. 166, 182–187 (2001).
Hombach, A. et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J. Immunol. 167, 6123–6131 (2001).
Maher, J., Brentjens, R. J., Gunset, G., Riviere, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nature Biotechnol. 20, 70–75 (2002).
Haynes, N. M. et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 100, 3155–3163 (2002). An important advance in the effectiveness of genetically redirected T cells is described, in which incorporation of a co-stimulatory domain into the cytoplasmic portion of a chimeric gene results in increased antitumour effects and even in the eradication of early subcutaneous tumours or lung metastases in mice.
Finney, H. M., Lawson, A. D., Bebbington, C. R. & Weir, A. N. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J. Immunol. 161, 2791–2797 (1998).
Finney, H. M., Akbar, A. N. & Lawson, A. D. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRζ chain. J. Immunol. 172, 104–113 (2004).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Geiger, T. L., Nguyen, P., Leitenberg, D. & Flavell, R. A. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood 98, 2364–2371 (2001).
Fitzer-Attas, C. J., Schindler, D. G., Waks, T. & Eshhar, Z. Harnessing Syk family tyrosine kinases as signaling domains for chimeric single chain of the variable domain receptors: optimal design for T cell activation. J. Immunol. 160, 145–154 (1998).
Bertram, E. M., Dawicki, W. & Watts, T. H. Role of T cell costimulation in anti-viral immunity. Semin. Immunol. 16, 185–196 (2004).
Vinay, D. S. & Kwon, B. S. Differential expression and costimulatory effect of 4-1BB (CD137) and CD28 molecules on cytokine-induced murine CD8+ TC1 and TC2 cells. Cell. Immunol. 192, 63–71 (1999).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different Notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Zheng, W. & Flavell, R. A. The transcription factor GATA-3 is necessary and sufficient for TH2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Szabo, S. J. et al. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell 100, 655–669 (2000).
Moretta, A. et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19, 197–223 (2001).
Ossendorp, F., Mengede, E., Camps, M., Filius, R. & Melief, C. J. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J. Exp. Med. 187, 693–702 (1998).
Mattes, J. et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Hombach, A. et al. An entirely humanized CD3 ζ-chain signaling receptor that directs peripheral blood T cells to specific lysis of carcinoembryonic antigen-positive tumor cells. Int. J. Cancer 88, 115–120 (2000).
Daly, T. et al. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 7, 284–291 (2000).
Weijtens, M. E., Willemsen, R. A., Valerio, D., Stam, K. & Bolhuis, R. L. Single chain Ig/γ gene-redirected human T lymphocytes produce cytokines, specifically lyse tumor cells, and recycle lytic capacity. J. Immunol. 157, 836–843 (1996).
Rossig, C., Bollard, C. M., Nuchtern, J. G., Merchant, D. A. & Brenner, M. K. Targeting of GD2-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. Int. J. Cancer 94, 228–236 (2001).
McGuinness, R. P. et al. Anti-tumor activity of human T cells expressing the CC49-ζ chimeric immune receptor. Hum. Gene Ther. 10, 165–173 (1999).
Hwu, P. et al. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with tumor necrosis factor-α cDNA for the gene therapy of cancer in humans. J. Immunol. 150, 4104–4115 (1993).
Itoh, Y. et al. Characterization of tumor-necrosis-factor-gene-transduced tumor-infiltrating lymphocytes from ascitic fluid of cancer patients: analysis of cytolytic activity, growth rate, adhesion molecule expression and cytokine production. Cancer Immunol. Immunother. 40, 95–102 (1995).
Crittenden, M. et al. Pharmacologically regulated production of targeted retrovirus from T cells for systemic antitumor gene therapy. Cancer Res. 63, 3173–3180 (2003).
Butz, E. A. & Bevan, M. J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Heemskerk, M. H. et al. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J. Exp. Med. 199, 885–894 (2004).
Kershaw, M. H., Westwood, J. A. & Hwu, P. Dual-specific T cells combine proliferation and antitumor activity. Nature Biotechnol. 20, 1221–1227 (2002). The authors of this report propose a new concept that involves dual-specific T cells to circumvent the poor immunogenicity of many TAAs. By combining tumour reactivity with specificity for a potent immunogen, in this case alloantigen, increased antitumour activity was observed in mice following adoptive transfer of dual-specific cells and allogeneic immunization.
Monsurro, V. et al. Kinetics of TCR use in response to repeated epitope-specific immunization. J. Immunol. 166, 5817–5825 (2001).
Yang, L., Qin, X. F., Baltimore, D. & Van Parijs, L. Generation of functional antigen-specific T cells in defined genetic backgrounds by retrovirus-mediated expression of TCR cDNAs in hematopoietic precursor cells. Proc. Natl Acad. Sci. USA 99, 6204–6209 (2002). This study shows the feasibility of providing a constant renewable source of T cells that are specific for ovalbumin by genetically modifying haemotopoietic stem cells. The introduction of tumour-specific TCRs into progenitor cells in this way could provide T cells that can affect existing malignant disease, as well as monitor the body for relapse in the long-term.
Cooper, L. J. et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 105, 1622–1631 (2005).
Kim, J. V., Latouche, J. B., Riviere, I. & Sadelain, M. The ABCs of artificial antigen presentation. Nature Biotechnol. 22, 403–410 (2004).
Liu, K. & Rosenberg, S. A. Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity. J. Immunol. 167, 6356–6365 (2001).
Cheng, L. E., Ohlen, C., Nelson, B. H. & Greenberg, P. D. Enhanced signaling through the IL-2 receptor in CD8+ T cells regulated by antigen recognition results in preferential proliferation and expansion of responding CD8+ T cells rather than promotion of cell death. Proc. Natl Acad. Sci. USA 99, 3001–3006 (2002).
Topp, M. S. et al. Restoration of CD28 expression in CD28− CD8+ memory effector T cells reconstitutes antigen-induced IL-2 production. J. Exp. Med. 198, 947–955 (2003).
Hooijberg, E. et al. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J. Immunol. 165, 4239–4245 (2000).
Lin, C. M. & Wang, F. H. Selective modification of antigen-specific CD4+ T cells by retroviral-mediated gene transfer and in vitro sensitization with dendritic cells. Clin. Immunol. 104, 58–66 (2002).
Charo, J. et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 65, 2001–2008 (2005). Increased survival is imparted to tumour-specific T cells by a transgene encoding the anti-apoptotic molecule BCL-2. Such apoptosis-resistant T cells are shown to have increased antitumour capacity in mice.
Eaton, D., Gilham, D. E., O'Neill, A. & Hawkins, R. E. Retroviral transduction of human peripheral blood lymphocytes with Bcl-XL promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 9, 527–535 (2002).
Dotti, G. et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood 105, 4677–4684 (2005).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Huang, H., Li, F., Gordon, J. R. & Xiang, J. Synergistic enhancement of antitumor immunity with adoptively transferred tumor-specific CD4+ and CD8+ T cells and intratumoral lymphotactin transgene expression. Cancer Res. 62, 2043–2051 (2002).
Homey, B., Muller, A. & Zlotnik, A. Chemokines: agents for the immunotherapy of cancer? Nature Rev. Immunol. 2, 175–184 (2002).
Haghnegahdar, H. et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J. Leukoc. Biol. 67, 53–62 (2000).
Chuntharapai, A., Lee, J., Hebert, C. A. & Kim, K. J. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J. Immunol. 153, 5682–5688 (1994).
Kershaw, M. H. et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 13, 1971–1980 (2002). This is the original description of the manipulation of the migration properties of T cells by insertion of a gene that encodes a receptor for the tumour-associated chemokine CXCL1. Although only carried out in vitro , this study raises the possibility of genetically redirecting T cells to penetrate tumours in greater numbers.
Iellem, A. et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4+CD25+ regulatory T cells. J. Exp. Med. 194, 847–853 (2001).
Bollard, C. M. et al. Adapting a transforming growth factor β-related tumor protection strategy to enhance antitumor immunity. Blood 99, 3179–3187 (2002). A novel method of enabling tumour- or virus-specific T cells to resist the tumour-derived inhibitory factor TGF-β is presented in this in vitro study. Many tumour types secrete TG-β, so this strategy might allow tumour-specific T cells to maintain their tumour-destructive properties in the face of an otherwise immunosuppressive environment.
Wagner, H. J. et al. A strategy for treatment of Epstein–Barr virus-positive Hodgkin's disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 11, 81–91 (2004).
Hacein-Bey-Abina, S. et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 348, 255–256 (2003).
Li, Z. et al. Murine leukemia induced by retroviral gene marking. Science 296, 497 (2002).
Bonini, C. et al. Safety of retroviral gene marking with a truncated NGF receptor. Nature Med. 9, 367–369 (2003).
Bonini, C. et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276, 1719–1724 (1997). This article deals with controlling the persistence and activity of tumour-reactive T cells (after they have been transferred to patients) by insertion of a suicide gene into the T cells. If the genetically modified T cells begin to damage normal tissue or become malignant themselves, a drug can be administered that eliminates genetically modified T cells without harming normal T cells.
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Thomis, D. C. et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood 97, 1249–1257 (2001).
Rettinger, S. D. et al. Liver-directed gene therapy: quantitative evaluation of promoter elements by using in vivo retroviral transduction. Proc. Natl Acad. Sci. USA 91, 1460–1464 (1994).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Speiser, D. E. et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 186, 645–653 (1997).
Nomura, T. & Sakaguchi, S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr. Top. Microbiol. Immunol. 293, 287–302 (2005).
Gorelik, L. & Flavell, R. A. Transforming growth factor-β in T-cell biology. Nature Rev. Immunol. 2, 46–53 (2002).
Salazar-Onfray, F. Interleukin-10: a cytokine used by tumors to escape immunosurveillance. Med. Oncol. 16, 86–94 (1999).
Cebon, J. et al. Two Phase I studies of low dose recombinant human IL-12 with Melan-A and influenza peptides in subjects with advanced malignant melanoma. Cancer Immun. 3, 7–25 (2003).
Sadanaga, N. et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin. Cancer Res. 7, 2277–2284 (2001).
Lonchay, C. et al. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc. Natl Acad. Sci. USA 101 (Suppl. 2), 14631–14638 (2004).
Peterson, A. C., Harlin, H. & Gajewski, T. F. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J. Clin. Oncol. 21, 2342–2348 (2003).
Lau, R. et al. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J. Immunother. 24, 66–78 (2001).
Murphy, G. P. et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a Phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 38, 73–78 (1999).
Morse, M. A. et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin. Cancer Res. 5, 1331–1338 (1999).
Kono, K. et al. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin. Cancer Res. 8, 3394–3400 (2002).
Scheibenbogen, C. et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte–macrophage colony-stimulating factor in patients with metastatic melanoma. J. Immunother. 23, 275–281 (2000).
Slingluff, C. L. Jr et al. Clinical and immunologic results of a randomized Phase II trial of vaccination using four melanoma peptides either administered in granulocyte–macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J. Clin. Oncol. 21, 4016–4026 (2003).
Sato, Y. et al. A Phase I trial of cytotoxic T-lymphocyte precursor-oriented peptide vaccines for colorectal carcinoma patients. Br. J. Cancer 90, 1334–1342 (2004).
Gulley, J. L. et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 11, 3353–3362 (2005).
Vonderheide, R. H. et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin. Cancer Res. 10, 828–839 (2004).
Knutson, K. L., Schiffman, K., Cheever, M. A. & Disis, M. L. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin. Cancer Res. 8, 1014–1018 (2002).
Jager, E. et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl Acad. Sci. USA 97, 12198–12203 (2000).
Khleif, S. N. et al. A Phase I vaccine trial with peptides reflecting ras oncogene mutations of solid tumors. J. Immunother. 22, 155–165 (1999).
Gulley, J. et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 53, 109–117 (2002).
von Mehren, M. et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin. Cancer Res. 6, 2219–2228 (2000).
Zhu, M. Z., Marshall, J., Cole, D., Schlom, J. & Tsang, K. Y. Specific cytolytic T-cell responses to human CEA from patients immunized with recombinant avipox–CEA vaccine. Clin. Cancer Res. 6, 24–33 (2000).
Lienard, D. et al. Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA. Cancer Immun. 4, 4–23 (2004).
Svane, I. M. et al. Vaccination with p53-peptide-pulsed dendritic cells, of patients with advanced breast cancer: report from a Phase I study. Cancer Immunol. Immunother. 53, 633–641 (2004).
Hersey, P. et al. Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol. Immunother. 53, 125–134 (2004).
Cathcart, K. et al. A multivalent bcr–abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood 103, 1037–1042 (2004).
Haynes, N. M. et al. Rejection of syngeneic colon carcinoma by CTLs expressing single-chain antibody receptors codelivering CD28 costimulation. J. Immunol. 169, 5780–5786 (2002).
Teng, M. W., Kershaw, M. H., Moeller, M., Smyth, M. J. & Darcy, P. K. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum. Gene Ther. 15, 699–708 (2004).
Kershaw, M. H. et al. Generation of gene-modified T cells reactive against the angiogenic kinase insert domain-containing receptor (KDR) found on tumor vasculature. Hum. Gene Ther. 11, 2445–2452 (2000).
Ren-Heidenreich, L., Hayman, G. T. & Trevor, K. T. Specific targeting of EGP-2+ tumor cells by primary lymphocytes modified with chimeric T cell receptors. Hum. Gene Ther. 11, 9–19 (2000).
Yun, C. O., Nolan, K. F., Beecham, E. J., Reisfeld, R. A. & Junghans, R. P. Targeting of T lymphocytes to melanoma cells through chimeric anti-GD3 immunoglobulin T-cell receptors. Neoplasia 2, 449–459 (2000).
Lamers, C. H., Willemsen, R. A., Luider, B. A., Debets, R. & Bolhuis, R. L. Protocol for gene transduction and expansion of human T lymphocytes for clinical immunogene therapy of cancer. Cancer Gene Ther. 9, 613–623 (2002).
Cooper, L. J. et al. Enhanced anti-lymphoma efficacy of CD19 redirected influenza MP1-specific CTL's by co-transfer of T-cells modified to present influenza MP1. Blood 105, 1622–1631 (2004).
Dotti, G. et al. Human cytotoxic T-lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood 105, 4677–4684 (2005).
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Acknowledgements
We acknowledge support from the National Health and Medical Research Council (Australia), The Cancer Council Victoria (Australia) and the Susan G. Komen Breast Cancer Foundation (United States). We apologize to colleagues whose work might have been cited only indirectly through reviews, owing to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- TUMOUR-ASSOCIATED ANTIGEN
-
(TAA). An antigen that is expressed by tumour cells. These antigens belong to four main categories: overexpressed antigens that are also expressed in small amounts in some normal tissues; tissue-differentiation antigens, which are also expressed by non-malignant cells; mutated or aberrantly expressed molecules; and cancer-testis antigens, which are normally expressed in the testes and occasionally in the placenta.
- CENTRAL TOLERANCE
-
Self-tolerance that is created at the level of the central lymphoid organs. Developing T cells, in the thymus, and B cells, in the bone marrow, that strongly recognize self-antigen face deletion or marked suppression.
- PERIPHERAL TOLERANCE
-
The lack of self-responsiveness of mature lymphocytes in the periphery to specific antigens. These mechanisms control potentially self-reactive lymphocytes that have escaped central tolerance. Peripheral tolerance is associated with suppression of production of self-reactive antibodies by B cells and inhibition of self-reactive effector cells, such as cytotoxic T lymphocytes.
- HUMANIZATION
-
A process by which parts of molecules originating from other species are replaced by the homologous domains of human molecules, using recombinant DNA techniques.
- SINGLE-CHAIN VARIABLE FRAGMENT
-
A recombinant molecule that is composed of the variable regions of the heavy and light chains of immunoglobulin joined by a flexible oligopeptide linker.
- HAPTEN
-
A molecule that can bind antibody but cannot by itself elicit an immune response. Antibodies that are specific for a hapten can be generated when the hapten is chemically linked to a protein carrier that can elicit a T-cell response.
- IMMUNOEDITING
-
The process by which interaction of a heterogeneous population of tumour cells with the immune system generates tumour variants with reduced immunogenicity, which might therefore escape from immune responses.
- SIGNAL-MOTIF LIBRARY
-
A collection of cDNA clones that encodes various intracellular-signalling domains joined together randomly and ligated to an extracellular antigen-recognition domain. Individual cells displaying different signalling constructs are screened for optimal function.
- TELOMERES
-
Regions of highly repetitive DNA at the ends of linear eukaryotic chromosomes. They protect the ends of chromosomes from shortening on replication. Telomerase reverse transcriptase is a ribonucleoprotein enzyme that maintains the ends of chromosomes by adding a characteristic series of nucleotides to telomeres.
- SMALL INTERFERING RNA
-
(siRNA). Synthetic RNA molecules of 19–23 nucleotides that are used to 'knockdown' (that is, silence the expression of) a specific gene. This is known as RNA interference (RNAi) and is mediated by the sequence-specific degradation of mRNA.
- PATTERN-RECOGNITION RECEPTOR
-
(PRR). A host receptor (such as Toll-like receptors) that can sense pathogen-associated molecular patterns and initiate signalling cascades (which involve activation of nuclear factor-κB) that lead to an innate immune response.
- CD4+CD25+ REGULATORY T CELL
-
A specialized type of CD4+ T cell that can suppress the responses of other T cells. These cells provide a crucial mechanism for the maintenance of peripheral self-tolerance and are characterized by expression of CD25 (also known as the α-chain of the interleukin-2 receptor) and the transcription factor forkhead box P3 (FOXP3).
Rights and permissions
About this article
Cite this article
Kershaw, M., Teng, M., Smyth, M. et al. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol 5, 928–940 (2005). https://doi.org/10.1038/nri1729
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1729
This article is cited by
-
Correlation between schistosomiasis and CD8+ T cell and stromal PD-L1 as well as the different prognostic role of CD8+ T cell and PD-L1 in schistosomal-associated colorectal cancer and non-schistosomal-associated colorectal cancer
World Journal of Surgical Oncology (2021)
-
CTLA4-CD28 chimera gene modification of T cells enhances the therapeutic efficacy of donor lymphocyte infusion for hematological malignancy
Experimental & Molecular Medicine (2017)
-
High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients
Leukemia (2017)
-
Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV
Protein & Cell (2017)
-
Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment
Protein & Cell (2017)