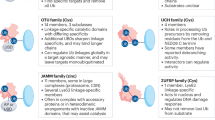
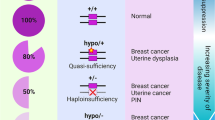
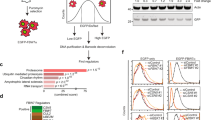
Abstract
Recent studies have shown that three major tumour-suppressor proteins undergo monoubiquitylation-mediated nuclear–cytoplasmic shuttling. Importantly, this mechanism has consequences for cancer and implies that proper localization is central to the function of tumour suppressors. This Progress article highlights recent efforts demonstrating that monoubiquitylation coupled to nuclear–cytoplasmic shuttling might be a novel regulatory mechanism that directly influences the function of tumour suppressors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Vogelstein, B. & Kinzler, K. W. Cancer genes and the pathways they control. Nature Med. 10, 789–799 (2004).
Hoeller, D., Hecker, C. M. & Dikic, I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nature Rev. Cancer 6, 776–788 (2006).
Wang, X. et al. NEDD4–1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128, 129–139 (2007).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Pickart, C. M. & Eddins, M. J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta. 1695, 55–72 (2004).
Kirkin, V. & Dikic, I. Role of ubiquitin- and Ubl-binding proteins in cell signaling. Curr. Opin. Cell Biol. 19, 199–205 (2007).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006).
Lloyd, A. C. p53: only ARF the story. Nature Cell Biol. 2, E48–E50 (2000).
Bode, A. M. & Dong, Z. Post-translational modification of p53 in tumorigenesis. Nature Rev. Cancer 4, 793–805 (2004).
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003).
Chipuk, J. E. & Green, D. R. Dissecting p53-dependent apoptosis. Cell Death. Differ. 13, 994–1002 (2006).
Marchenko, N. D., Wolff, S., Erster, S., Becker, K. & Moll, U. M. Monoubiquitylation promotes mitochondrial p53 translocation. EMBO J. 26, 923–934 (2007).
Becker, K., Marchenko, N. D., Maurice, M. & Moll, U. M. Hyperubiquitylation of wild-type p53 contributes to cytoplasmic sequestration in neuroblastoma. Cell Death. Differ. 23 March 2007 (Epub ahead of print).
Hirano, Y. & Ronai, Z. A new function for p53 ubiquitination. Cell 127, 675–677 (2006).
Iwakuma, T. & Lozano, G. MDM2, an introduction. Mol. Cancer Res. 1, 993–1000 (2003).
Greer, E. L. & Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 (2005).
Lehmann, O. J., Sowden, J. C., Carlsson, P., Jordan, T. & Bhattacharya, S. S. Fox's in development and disease. Trends Genet. 19, 339–344 (2003).
Paik, J. H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Takaishi, H. et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc. Natl Acad. Sci. USA 96, 11836–11841 (1999).
Biggs, W. H. 3rd, Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl Acad. Sci. USA 96, 7421–7426 (1999).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA 102, 1649–1654 (2005).
Essers, M. A. et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 (2004).
Oh, S. W. et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl Acad. Sci. USA 102, 4494–4499 (2005).
van der Horst, A. et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nature Cell Biol. 8, 1064–1073 (2006).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 8, 1169–1178 (1998).
Stambolic, V. et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95, 29–39 (1998).
Haas-Kogan, D. et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8, 1195–1198 (1998).
Maehama, T. & Dixon, J. E. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 9, 125–128 (1999).
Eng, C. PTEN: one gene, many syndromes. Hum. Mutat. 22, 183–198 (2003).
Gil, A., Andres-Pons, A. & Pulido, R. Nuclear PTEN: a tale of many tails. Cell Death Differ. 14, 395–399 (2007).
Lian, Z. & Di Cristofano, A. Class reunion: PTEN joins the nuclear crew. Oncogene 24, 7394–7400 (2005).
Shen, W. H. et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128, 157–170 (2007).
Georgescu, M. M., Kirsch, K. H., Akagi, T., Shishido, T. & Hanafusa, H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl Acad. Sci. USA 96, 10182–10187 (1999).
Torres, J. & Pulido, R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J. Biol. Chem. 276, 993–998 (2001).
Vazquez, F., Ramaswamy, S., Nakamura, N. & Sellers, W. R. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell Biol. 20, 5010–5018 (2000).
Trotman, L. C. et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128, 141–156 (2007).
Lee, J. O. et al. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99, 323–334 (1999).
Acknowledgements
We would like to thank all the members of the Pandolfi lab who contributed to these ideas. We are grateful to J.G. Clohessy and J. Kotsopoulos for critically reviewing the manuscript. We apologize to those colleagues whose work could not be included owing to space limitations. The work of P.P.P. is supported by the US National Cancer Institute, L.S. is supported by a Long Term Fellowship from the Human Frontier Science Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Salmena, L., Pandolfi, P. Changing venues for tumour suppression: balancing destruction and localization by monoubiquitylation. Nat Rev Cancer 7, 409–413 (2007). https://doi.org/10.1038/nrc2145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2145
This article is cited by
-
MG53 suppresses tumor progression and stress granule formation by modulating G3BP2 activity in non-small cell lung cancer
Molecular Cancer (2021)
-
Post translational modification of Parkin
Biology Direct (2017)
-
BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP
Leukemia (2014)
-
MCT-1 expression and PTEN deficiency synergistically promote neoplastic multinucleation through the Src/p190B signaling activation
Oncogene (2014)
-
USP2a alters chemotherapeutic response by modulating redox
Cell Death & Disease (2013)