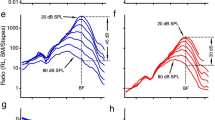
Abstract
The organs of the vestibular, auditory and lateral line systems rely on a common strategy for the stimulation of their primary receptors, the hair cells: stimuli induce shear between hair cell epithelia and accessory structures to which hair bundles, the hair cells' mechanosensitive organelles, are attached. The inner hair cells of the cochlea, whose hair bundles are not attached to the overlying tectorial membrane, are a notable exception. Because their hair bundles are not restrained, they undergo significant Brownian motion, a characteristic traditionally thought to blunt the sensitivity of hearing. Contrary to this view, the work reported here indicates that Brownian motion of the hair bundle serves to enhance the sensitivity of mechanoelectrical transduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
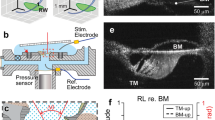
References
de Vries, H. L. Brownian movement and hearing. Physica 14, 48–60 (1948).
Corey, D. P. & Hudspeth, A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. 3, 962–976 (1983).
Harris, G. G. Brownian motion in the cochlear partition. J. Acoust. Soc. Am. 44, 176–186 ( 1968).
Wiesenfeld, K. & Moss, F. Stochastic resonance and the benefits of noise: from ice ages to crayfish and SQUIDs. Nature 373, 33–36 (1995).
McNamara, B. & Wiesenfeld, K. Theory of stochastic resonance. Phys. Rev. A 39, 4854– 4869 (1989).
Bezrukov, S. M. & Vodyanov, I. Noise-induced enhancement of signal transduction across voltage-dependent ion channels. Nature 378, 362–364 (1995).
Collins, J. J., Imhoff, T. T. & Grigg, P. Noise-enhanced information transmission in rat SA1 cutaneous mechanoreceptors via aperiodic stochastic resonance. J. Neurophysiol. 76, 642–645 ( 1996).
Levin, J. E. & Miller, J. P. Broadband neural encoding in the cricket cercal sensory system enhanced by stochastic resonance. Nature 380, 165–168 ( 1996).
Douglass, J. K., Wilkens, L., Pantazelou, E. & Moss, F. Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature 365, 337– 340 (1993).
Narins, P. M., Benedix, J. H. Jr., & Moss, F. Can increasing temperature improve information transfer in the anuran peripheral auditory system? Aud. Neurosci. 3, 389–400 (1997).
Svrcek-Seiler, W. A., Gebeshuber, I. C., Rattay, F., Biro, T. S. & Markum, H. Micromechanical models for the Brownian motion of hair cell stereocilia. J. Theor. Biol. (in press).
Lindeman, H. H., Ades, H. W., Bredberg, G. & Engström, H. The sensory hairs and the tectorial membrane in the development of the cat's organ of Corti. Acta Otolaryngol. 72, 229 –242 (1972).
Dallos, P. Response characteristics of mammalian cochlear hair cells. J. Neurosci. 5, 1591–1608 ( 1985).
Dallos, P., Billone, M. C., Durrant, J. D., Wang, C. & Raynor, S. Cochlear inner and outer hair cells: functional differences. Science 177, 356 –358 (1985).
Ruggero, M. Responses to sound of the basilar membrane of the mammalian cochlea. Curr. Opin. Neurobiol. 2, 449–456 (1992).
Denk, W. & Webb, W. W. Forward and reverse transduction at the limit of sensitivity studied by correlating electrical and mechanical fluctuations in frog saccular hair cells. Hear. Res. 60, 89–102 (1992).
Denk, W., Webb, W. W. & Hudspeth, A. J. Mechanical properties of sensory hair bundles are reflected in their Brownian motion measured with a laser differential interferometer. Proc. Natl Acad. Sci. USA 86, 5371– 5375 (1989).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 ( 1981).
Howard, J. & Hudspeth, A. J. Mechanical relaxation of the hair bundle mediates adaptation in mechanoelectrical transduction by the bullfrog's saccular hair cell. Proc. Natl Acad. Sci. USA 84, 3064–3068 (1987).
Howard, J. & Ashmore, J. F. Stiffness of sensory hair bundles in the sacculus of the frog. Hear. Res. 23, 93–104 (1986).
Atkins, P. W. in Physical Chemistry, (Oxford University Press, Oxford, 1978).
Howard, J., Roberts, W. M. & Hudspeth, A. J. Mechanoelectrical transduction by hair cells. Ann. Rev. Biophys. and Biophys. Chem. 17, 99– 124 (1988).
Russell, I. J., Richardson, G. P. & Cody, A. R. Mechanosensitivity of mammalian auditory hair cells in vitro. Nature 321, 517– 519 (1986).
Russell, I. J., Kossl, M. & Richardson, G. P. Nonlinear mechanical responses of mouse cochlear hair bundles. Proc. R. Soc. Lond. B 250, 217–227 (1992).
Howard, J. & Hudspeth, A. J. Gating compliance associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron 1, 189– 199 (1988).
Pickles, J. O., Comis, S. D. & Osborne, M. P. Cross-links between sterocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112 ( 1984).
Assad, J. A., Hacohen, N. & Corey, D. P. Voltage dependence of adaptation and active bundle movement in bullfrog saccular hair cells. Proc. Natl Acad. Sci. USA 86, 2918–2922 ( 1989).
Acknowledgements
We thank Tim Cope and Ray Dingledine for critically reading the manuscript, Richard Jacobs for technical advice, and Mario Ruggero, Ille Gebeshuber and Peter Jung for discussions. This work was supported by the NIDCD and by a grant from the Emory/Georgia Tech. Consortium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaramillo, F., Wiesenfeld, K. Mechanoelectrical transduction assisted by Brownian motion: a role for noise in the auditory system. Nat Neurosci 1, 384–388 (1998). https://doi.org/10.1038/1597
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/1597
This article is cited by
-
Mechanisms underlying treatment effects of vestibular noise stimulation on postural instability in patients with bilateral vestibulopathy
Journal of Neurology (2023)
-
A biomimetic neural encoder for spiking neural network
Nature Communications (2021)
-
No evidence for stochastic resonance effects on standing balance when applying noisy galvanic vestibular stimulation in young healthy adults
Scientific Reports (2021)
-
Inconsistent effects of stochastic resonance on human auditory processing
Scientific Reports (2020)
-
Augmenting Global Coherence in EEG Signals with Binaural or Monaural Noises
Brain Topography (2020)