Abstract
The presence of antibodies to TSH receptor (TSHR) is the hallmark of Graves disease (GD). These antibodies mimic the action of TSH, resulting in TSHR stimulation and hyperthyroidism, and have been associated with GD-associated extrathyroidal manifestations. TSH binding inhibition assays and bioassays for measurement of TSHR antibody levels have been used for clinical and research purposes. In the former, inhibition of TSH binding to purified or recombinant TSHR by a patient's immunoglobulins is measured by radioactive or chemiluminescent techniques. In the latter, cyclic AMP production is measured by use of radioimmunoassays or chemiluminescent methods in cells natively or artificially expressing TSHR. In this Review, the different techniques used for the detection of antibodies to TSHR are discussed, together with the clinical applications of antibody measurement, including diagnosis of GD and Graves ophthalmopathy. Prediction of relapse after medical treatment and the clinical course of Graves ophthalmopathy are also addressed.
Key Points
-
Antibodies to TSH receptor (TSHR) have key roles in the pathogenesis of Graves disease and have been associated with its extrathyroidal complications
-
TSHR antibodies might have thyroid-stimulating properties, resulting in hyperthyroidism, or blocking activity, causing hypothyroidism
-
TSH binding inhibiting immunoglobulin assays detect the broad range of TSHR antibodies but cannot differentiate between those with stimulating and blocking activity; bioassays can make this differentiation
-
TSH binding inhibiting immunoglobulin assays are commercially available and, unlike bioassays, are easy to perform; second-generation assays have high sensitivity and specificity for diagnostic purposes
-
The main clinical uses for measurement of antibodies to TSHR is diagnosis of atypical cases of Graves disease, prediction of neonatal thyroid dysfunction in pregnant women with this condition and identification of Graves ophthalmopathy in euthyroid individuals
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Adams DD (1988) Long-acting thyroid stimulator: how receptor autoimmunity was discovered. Autoimmunity 1: 3–9
Rapoport B et al. (1998) The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev 19: 673–716
Davies TF et al. (2005) Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest 115: 1972–1983
Ando T et al. (2005) Thyrotropin receptor antibodies: new insights into their actions and clinical relevance. Best Pract Res Clin Endocrinol Metab 19: 33–52
Smith BR and Hall R (1974) Thyroid-stimulating immunoglobulins in Graves' disease. Lancet 2: 427–431
Costagliola S et al. (1999) Second generation assay for thyrotropin receptor antibodies has superior diagnostic sensitivity for Graves' disease. J Clin Endocrinol Metab 84: 90–97
Villalta D et al. (2004) Analytical and diagnostic accuracy of “second generation” assays for thyrotrophin receptor antibodies with radioactive and chemiluminescent tracers. J Clin Pathol 57: 378–382
Paunkovic J and Paunkovic N (2006) Does autoantibody-negative Graves' disease exist? A second evaluation of the clinical diagnosis. Horm Metab Res 38: 53–56
Preissner CM et al. (2003) Comparison of thyrotropin-receptor antibodies measured by four commercially available methods with a bioassay that uses Fisher rat thyroid cells. Clin Chem 49: 1402–1404
Smith BR et al. (2004) A new assay for thyrotropin receptor autoantibodies. Thyroid 14: 830–835
Kamijo K et al. (2005) Clinical evaluation of 3rd generation assay for thyrotropin receptor antibodies: the M22-biotin-based ELISA initiated by Smith. Endocr J 52: 525–529
Atger M et al. (1999) Autoantibodies interacting with purified native thyrotropin receptor. Eur J Biochem 265: 1022–1031
Nagayama Y et al. (1991) Binding domains of stimulatory and inhibitory thyrotropin (TSH) receptor autoantibodies determined with chimeric TSH-lutropin/chorionic gonadotropin receptors. J Clin Invest 88: 336–340
Tahara K et al. (1991) Immunoglobulins from Graves' disease patients interact with different sites on TSH receptor/LH-CG receptor chimeras than either TSH or immunoglobulins from idiopathic myxedema patients. Biochem Biophys Res Commun 179: 70–77
Costagliola S et al. (2004) Delineation of the discontinuous-conformational epitope of a monoclonal antibody displaying full in vitro and in vivo thyrotropin activity. Mol Endocrinol 18: 3020–3034
Minich WB et al. (2004) Antibodies to TSH-receptor in thyroid autoimmune disease interact with monoclonal antibodies whose epitopes are broadly distributed on the receptor. Clin Exp Immunol 136: 129–136
Morgenthaler NG et al. (2003) Affinity purification and diagnostic use of TSH receptor autoantibodies from human serum. Mol Cell Endocrinol 212: 73–79
Morgenthaler NG et al. (2007) Stimulating and blocking thyroid-stimulating hormone (TSH) receptor autoantibodies from patients with Graves' disease and autoimmune hypothyroidism have very similar concentration, TSH receptor affinity, and binding sites. J Clin Endocrinol Metab 92: 1058–1065
Zakarija M and McKenzie JM (1973) Effect of thyrotropin and of LATS on mouse thyroid cyclic AMP. Metabolism 22: 1185–1191
Stockle G et al. (1981) Micromethod of human thyrocyte cultures for detection of thyroid-stimulating antibodies and thyrotrophin. Acta Endocrinol (Copenh) 97: 369–375
Kasagi K et al. (1982) A new in vitro assay for human thyroid stimulator using cultured thyroid cells: effect of sodium chloride on adenosine 3',5'-monophosphate increase. J Clin Endocrinol Metab 54: 108–114
Vitti P et al. (1993) Detection of thyroid-stimulating antibody using Chinese hamster ovary cells transfected with cloned human thyrotropin receptor. J Clin Endocrinol Metab 76: 499–503
Chiovato L et al. (1994) Detection of antibodies blocking thyrotropin effect using Chinese hamster ovary cells transfected with the cloned human TSH receptor. J Endocrinol Invest 17: 809–816
Murakami M et al. (1995) Clinical usefulness of thyroid-stimulating antibody measurement using Chinese hamster ovary cells expressing human thyrotropin receptors. Eur J Endocrinol 133: 80–86
Takano T et al. (1997) Detection of thyroid-stimulating antibody using frozen stocks of Chinese hamster ovary cells transfected with cloned human thyrotropin receptor. Endocr J 44: 431–435
Watson PF et al. (1998) A new chemiluminescent assay for the rapid detection of thyroid stimulating antibodies in Graves' disease. Clin Endocrinol (Oxf) 49: 577–581
Hovens GC et al. (2006) A bioluminescence assay for thyrotropin receptor antibodies predicts serum thyroid hormone levels in patients with de novo Graves' disease. Clin Endocrinol (Oxf) 64: 429–435
Evans C et al. (1999) Development of a luminescent bioassay for thyroid stimulating antibodies. J Clin Endocrinol Metab 84: 374–377
Da Costa CR and Johnstone AP (1998) Production of the thyrotrophin receptor extracellular domain as a glycosylphosphatidylinositol-anchored membrane protein and its interaction with thyrotrophin and autoantibodies. J Biol Chem 273: 11874–11880
Costagliola S et al. (1998) Production of bioactive amino-terminal domain of the thyrotropin receptor via insertion in the plasma membrane by a glycosylphosphatidylinositol anchor. FEBS Lett 436: 427–433
Chazenbalk GD et al. (2002) Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest 110: 209–217
Chazenbalk GD et al. (2004) Thyroid stimulation does not require antibodies with identical epitopes but does involve recognition of a critical conformation at the N terminus of the thyrotropin receptor A-subunit. J Clin Endocrinol Metab 89: 1788–1793
Minich WB et al. (2004) A coated tube assay for the detection of blocking thyrotropin receptor autoantibodies. J Clin Endocrinol Metab 89: 352–356
Shibayama K et al. (2005) Assays for thyroid-stimulating antibodies and thyrotropin-binding inhibitory immunoglobulins in children with Graves' disease. Endocr J 52: 505–510
Inui T et al. (1998) Increase of thyroid stimulating activity in Graves' immunoglobulin-G by high polyethylene glycol concentrations using porcine thyroid cell assay. Thyroid 8: 319–325
Schwarz-Lauer L et al. (2002) Evidence for a simplified view of autoantibody interactions with the thyrotropin receptor. Thyroid 12: 115–120
Davies TF et al. (1998) Thyroid controversy—stimulating antibodies. J Clin Endocrinol Metab 83: 3777–3785
Gilmour J et al. (2000) The quantitative measurement of autoantibodies to thyroglobulin and thyroid peroxidase by automated microparticle based immunoassays in Hashimoto's disease, Graves' disease and a follow-up study on postpartum thyroid disease. Clin Lab 46: 57–61
Guilhem I et al. (2006) Differential evolution of thyroid peroxidase and thyrotropin receptor antibodies in Graves' disease: thyroid peroxidase antibody activity reverts to pretreatment level after carbimazole withdrawal. Thyroid 16: 1041–1045
Lavard L et al. (2000) Prevalence of thyroid peroxidase, thyroglobulin and thyrotropin receptor antibodies in a long-term follow-up of juvenile Graves' disease. Autoimmunity 32: 167–172
Feldt-Rasmussen U et al. (1994) Meta-analysis evaluation of the impact of thyrotropin receptor antibodies on long term remission after medical therapy of Graves' disease. J Clin Endocrinol Metab 78: 98–102
Zimmermann-Belsing T et al. (2002) Use of the 2nd generation TRAK human assay did not improve prediction of relapse after antithyroid medical therapy of Graves' disease. Eur J Endocrinol 146: 173–177
Schott M et al. (2004) Levels of autoantibodies against human TSH receptor predict relapse of hyperthyroidism in Graves' disease. Horm Metab Res 36: 92–96
Quadbeck B et al. (2005) Sensitive thyrotropin and thyrotropin-receptor antibody determinations one month after discontinuation of antithyroid drug treatment as predictors of relapse in Graves' disease. Thyroid 15: 1047–1054
Cappelli C et al. (2007) Prognostic value of thyrotropin receptor antibodies (TRAb) in Graves' disease: a 120 months prospective study. Endocr J 54: 713–720
Weetman AP (2000) Graves' disease. N Engl J Med 343: 1236–1248
Noh JY et al. (2000) Thyroid-stimulating antibody is related to Graves' ophthalmopathy, but thyrotropin-binding inhibitor immunoglobulin is related to hyperthyroidism in patients with Graves' disease. Thyroid 10: 809–813
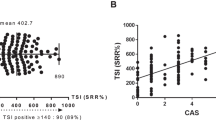
Gerding MN et al. (2000) Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf) 52: 267–271
Morris JC III et al. (1988) Clinical utility of thyrotropin-receptor antibody assays: comparison of radioreceptor and bioassay methods. Mayo Clin Proc 63: 707–717
Khoo TK and Bahn RS (2007) Pathogenesis of Graves' ophthalmopathy: the role of autoantibodies. Thyroid 17: 1013–1018
Eckstein AK et al. (2006) Thyrotropin receptor autoantibodies are independent risk factors for Graves' ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab 91: 3464–3470
Khoo DH et al. (2000) Graves' ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid 10: 1093–1100
Fisher DA (1997) Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol 40: 16–31
Chan GW and Mandel SJ (2007) Therapy insight: management of Graves' disease during pregnancy. Nat Clin Pract Endocrinol Metab 3: 470–478
Nayak B and Hodak SP (2007) Hyperthyroidism. Endocrinol Metab Clin North Am 36: 617–56
Weetman AP (1994) The immunomodulatory effects of antithyroid drugs. Thyroid 4: 145–146
Carella C et al. (2006) Serum thyrotropin receptor antibodies concentrations in patients with Graves' disease before, at the end of methimazole treatment, and after drug withdrawal: evidence that the activity of thyrotropin receptor antibody and/or thyroid response modify during the observation period. Thyroid 16: 295–302
McGregor AM et al. (1979) Effect of irradiation on thyroid-autoantibody production. Lancet 2: 442–444
Bartalena L et al. (1998) Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 338: 73–78
Andrade VA et al. (2004) Serum thyrotropin-receptor autoantibodies levels after I therapy in Graves' patients: effect of pretreatment with methimazole evaluated by a prospective, randomized study. Eur J Endocrinol 151: 467–474
Mukhtar ED et al. (1975) Relation of thyroid-stimulating immunoglobulins to thyroid function and effects of surgery, radioiodine, and antithyroid drugs. Lancet 1: 713–715
Acknowledgements
RA Ajjan is funded by a Department of Health Clinician Scientist Award-UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ajjan, R., Weetman, A. Techniques to quantify TSH receptor antibodies. Nat Rev Endocrinol 4, 461–468 (2008). https://doi.org/10.1038/ncpendmet0886
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0886
This article is cited by
-
Usefulness of TSH receptor antibodies as biomarkers for Graves’ ophthalmopathy: a systematic review
Journal of Endocrinological Investigation (2018)
-
Diagnosis and management of Graves disease: a global overview
Nature Reviews Endocrinology (2013)
-
Attitudes of US medical trainees towards neurology education: "Neurophobia" - a global issue
BMC Medical Education (2010)