Abstract
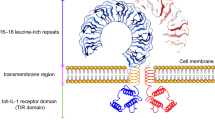
The innate immune system detects highly conserved, relatively invariant structural motifs of pathogens. Toll-like receptors (TLRs) have been identified as the primary innate immune receptors. TLRs distinguish between different patterns of pathogens and activate a rapid innate immune response; however, TLRs can also be activated by host-derived molecules. In addition to being expressed in immune cells, TLRs are expressed in other tissues, such as those of the cardiovascular system. TLRs could, therefore, be a key link between cardiovascular disease development and the immune system. Indeed, evidence that TLR activation contributes to the development and progression of atherosclerosis, cardiac dysfunction in sepsis, and congestive heart failure, is convincing. Although much has been learned about TLR activation in cellular components of the cardiovascular system, the role individual TLR family members have in the pathophysiology of cardiovascular diseases and hence in clinical practice remains to be defined. Here we review the rapid progress that has been made in this field, which has improved our understanding of vascular as well as myocardial TLR function in basic and clinical science.
Key Points
-
The innate immune system detects evolutionary highly conserved structural motifs of pathogens; Toll-like receptors (TLRs) are a family of pattern recognition receptors with central importance in the innate immune response
-
TLRs are expressed in vascular as well as myocardial cells
-
In myocardial diseases TLRs might be important in septic and toxic cardiomyopathy as well as hypertrophy and heart failure; this evidence comes almost exclusively from basic science data
-
A considerable amount of evidence links the pathogenesis of atherosclerosis with TLR signaling, through association studies of TLR4 polymorphisms and myocardial infarction risk, direct activation of TLRs after myocardial infarction, and experimental in vivo studies demonstrating decreased atherosclerosis development in TLR knockout mice
-
The function of TLRs in clinical practice remains to be defined
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frantz S et al. (2005) Innate immunity and the heart. Curr Pharm Des 11: 1279–1290
Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54: 1–13
Akira S et al. (2006) Pathogen recognition and innate immunity. Cell 124: 783–801
Medzhitov R et al. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397
Matzinger P (2002) The danger model: a renewed sense of self. Science 296: 301–305
Shi Y et al. (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521
Gallucci S et al. (1999) Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5: 1249–1255
Kawasaki K et al. (2000) Mouse toll-like receptor 4. MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem 275: 2251–2254
Vabulas RM et al. (2001) Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 276: 31332–31339
Okamura Y et al. (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233
Scheibner KA et al. (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281
Kaisho T and Akira S (2006) Toll-like receptor function and signaling. J Allergy Clin Immunol 117: 979–987
Akira S and Takeda K (1998) Toll-like receptor signalling. Nat Rev Immunol 4: 499–511
Medzhitov R et al. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 2: 253–258
Frantz S et al. (1999) Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest 104: 271–280
Re F and Strominger JL (2001) Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem 276: 37692–37699
Harari OA et al. (2006) Absence of TRAM restricts Toll-like receptor 4 signaling in vascular endothelial cells to the MyD88 pathway. Circ Res 98: 1134–1140
Libby P (2002) Inflammation in atherosclerosis. Nature 420: 868–874
Saikku P et al. (1988) Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet 2: 983–986
Grayston JT et al. (2005) Azithromycin for the secondary prevention of coronary events. N Engl J Med 352: 1637–1645
Arbour NC et al. (2000) TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 25: 187–191
Kiechl S et al. (2002) Toll-like receptor 4 polymorphisms and atherogenesis. N Engl J Med 347: 185–192
Erridge C et al. (2003) Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. J Exp Med 197: 1787–1791
Labrum R et al. (2007) Toll receptor polymorphisms and carotid artery intima-media thickness. Stroke 38: 1179–1184
Ameziane N et al. (2003) Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol 23: e61–e64
Boekholdt SM et al. (2003) Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation 107: 2416–2421
Koch W et al. (2006) Toll-like receptor 4 gene polymorphisms and myocardial infarction: no association in a Caucasian population. Eur Heart J 27: 2524–2529
Lin YC et al. (2005) Toll-like receptor 4 gene C119A but not Asp299Gly polymorphism is associated with ischemic stroke among ethnic Chinese in Taiwan. Atherosclerosis 180: 305–309
Satoh M et al. (2006) Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol 109: 226–234
Methe H et al. (2005) Expansion of circulating Toll-like receptor 4-positive monocytes in patients with acute coronary syndrome. Circulation 111: 2654–2661
Edfeldt K et al. (2002) Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105: 1158–1161
Xu XH et al. (2001) Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104: 3103–3108
Doherty TM et al. (2006) TLR signaling and trapped vascular dendritic cells in the development of atherosclerosis. Trends Immunol 27: 222–227
Dunzendorfer S et al. (2004) Flow-dependent regulation of endothelial Toll-like receptor 2 expression through inhibition of SP1 activity. Circ Res 95: 684–691
Bjorkbacka H et al. (2004) Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med 10: 416–421
Michelsen KS et al. (2004) Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA 101: 10679–10684
Hollestelle SC et al. (2004) Toll-like receptor 4 is involved in outward arterial remodeling. Circulation 109: 393–398
Mullick AE et al. (2005) Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 115: 3149–3156
Schoneveld AH et al. (2005) Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res 66: 162–169
Shishido T et al. (2006) Central role of endogenous Toll-like receptor-2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem Biophys Res Commun 345: 1446–1453
Frantz S et al. (2005) Innate immunity and angiogenesis. Circ Res 96: 15–26
Pollet I et al. (2003) Bacterial lipopolysaccharide directly induces angiogenesis through TRAF6-mediated activation of NF-kappaB and c-Jun N-terminal kinase. Blood 102: 1740–1742
Montesinos MC et al. (2002) Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol 160: 2009–2018
Pinhal-Enfield G et al. (2003) An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and adenosine A(2A) receptors. Am J Pathol 163: 711–721
Montesinos MC et al. (2004) Adenosine A(2A) receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am J Pathol 164: 1887–1892
Frantz S et al. (2001) Role of TLR-2 in the activation of nuclear factor-kappa B by oxidative stress in cardiac myocytes. J Biol Chem 276: 5197–5203
Zhu X et al. (2006) MyD88 and NOS2 are essential for Toll-like receptor 4-mediated survival effect in cardiomyocytes. Am J Physiol Heart Circ Physiol 291: H1900–H1909
Thomas JA et al. (2003) IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protects against contractile dysfunction. Am J Physiol Heart Circ Physiol 285: H597–H606
Nemoto S et al. (2002) Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol 282: H2316–H2323
Tavener SA et al. (2004) Immune cell Toll-like receptor 4 is required for cardiac myocyte impairment during endotoxemia. Circ Res 95: 700–707
Knuefermann P et al. (2004) Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation 110: 3693–3698
Chong AJ et al. (2004) Toll-like receptor 4 mediates ischemia/reperfusion injury of the heart. J Thorac Cardiovasc Surg 128: 170–179
Oyama J et al. (2004) Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789
Frantz S et al. (2007) Tissue-specific effects of the nuclear factor kappa B subunit p50 on myocardial ischemia-reperfusion injury. Am J Pathol [doi:10.2353/ajpath.2007.061042]
Ertl G and Frantz S (2005) Healing after myocardial infarction. Cardiovasc Res 66: 22–32
Birks EJ et al. (2004) Increased toll-like receptor 4 in the myocardium of patients requiring left ventricular assist devices. J Heart Lung Transplant 23: 228–235
Frantz S et al. (2006) Absence of NF-kappaB subunit p50 improves heart failure after myocardial infarction. FASEB J 20: 1918–1920
Tillmanns J et al. (2006) Caught in the act: in vivo molecular imaging of the transcription factor NF-kappaB after myocardial infarction. Biochem Biophys Res Commun 342: 773–774
Chao W et al. (2002) Importance of FADD signaling in serum deprivation- and hypoxia-induced cardiomyocyte apoptosis. J Biol Chem 277: 31639–31645
Shishido T et al. (2003) Toll-like receptor-2 modulates ventricular remodeling after myocardial infarction. Circulation 108: 2905–2910
Ha T et al. (2005) Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res 68: 224–234
Zhang D et al. (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 6: 556–563
Ha T et al. (2006) Blockade of MyD88 attenuates cardiac hypertrophy and decreases cardiac myocyte apoptosis in pressure overload-induced cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 290: H985–H994
Freund C et al. (2005) Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 111: 2319–2325
Nozaki N et al. (2004) Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110: 2869–2874
Ulevitch RJ (2004) Therapeutics targeting the innate immune system. Nat Rev Immunol 4: 512–520
Vanags D et al. (2006) Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet 368: 855–863
Anker SD and Coats AJ (2002) How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol 86: 123–130
Mahaffey KW et al. (2003) Effect of pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to fibrinolysis in acute myocardial infarction: the COMPlement inhibition in myocardial infarction treated with thromboLYtics (COMPLY) trial. Circulation 108: 1176–1183
Netea MG et al. (2004) Toll-like receptor-4 Asp299Gly polymorphism does not influence progression of atherosclerosis in patients with familial hypercholesterolaemia. Eur J Clin Invest 34: 94–99
Norata GD et al. (2005) Effect of the Toll-like receptor 4 (TLR-4) variants on intima-media thickness and monocyte-derived macrophage response to LPS. J Intern Med 258: 21–27
Yang IA et al. (2003) TLR4 Asp299Gly polymorphism is not associated with coronary artery stenosis. Atherosclerosis 170: 187–190
Edfeldt K et al. (2004) Association of hypo-responsive toll-like receptor 4 variants with risk of myocardial infarction. Eur Heart J 25: 1447–1453
Morange PE et al. (2004) TLR4/Asp299Gly, CD14/C-260T, plasma levels of the soluble receptor CD14 and the risk of coronary heart disease: The PRIME Study. Eur J Hum Genet 12: 1041–1049
Hamann L et al. (2005) A frequent toll-like receptor (TLR)-2 polymorphism is a risk factor for coronary restenosis. J Mol Med 83: 478–485
Holloway JW et al. (2005) Variation in the toll-like receptor 4 gene and susceptibility to myocardial infarction. Pharmacogenet Genomics 15: 15–21
Zee RY et al. (2005) Toll-like receptor 4 Asp299Gly gene polymorphism and risk of atherothrombosis. Stroke 36: 154–157
Acknowledgements
Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape–accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Frantz, S., Ertl, G. & Bauersachs, J. Mechanisms of Disease: Toll-like receptors in cardiovascular disease. Nat Rev Cardiol 4, 444–454 (2007). https://doi.org/10.1038/ncpcardio0938
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0938
This article is cited by
-
The expression of TLR2 and TLR4 in the kidneys and heart of mice infected with Acanthamoeba spp.
Parasites & Vectors (2020)
-
Emerging roles of Toll-like receptor 9 in cardiometabolic disorders
Inflammation and Regeneration (2020)
-
Involvement of Toll-like Receptor 4 in Neutrophil-Mediated Inflammation, Oxidative Stress and Tissue Damage Induced by Scorpion Venom
Inflammation (2020)
-
Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-κB signaling pathway
Scientific Reports (2018)
-
Tumor Necrosis Factor-α in Heart Failure: an Updated Review
Current Cardiology Reports (2018)