Abstract
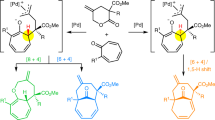
Cycloadditions that involve more than six π electrons are termed higher-order cycloadditions and are an excellent tool for solving complex synthetic challenges, as they provide direct access to polycyclic scaffolds that contain medium-sized rings. They have interesting synthetic potential for the discovery of new bioactive molecules and in natural product synthesis. It is peculiar that stereocontrolled [8+2] and [6+4] cycloadditions have been largely neglected for the past 50 years. Here we demonstrate a cross-dienamine activation of 2-cyclopentenone and the unprecedented endocyclic linear-dienamine activation of 2-cyclohexenones and 2-cycloheptenones. These dienamine intermediates undergo aminocatalytic stereoselective [8+2], [6+4] and formal [4+2] cycloadditions with various heptafulvenes. The periselectivities of the cycloadditions are controlled based on the ring size of the 2-cycloalkenones and the substitution patterns of the heptafulvenes. The chiral products obtained undergo various chemical and photochemical single-step transformations that give access to other classes of all-carbon polycyclic scaffolds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Woodward, R. B. & Hoffmann, R. Stereochemistry of electrocyclic reactions. J. Am. Chem. Soc. 87, 395–397 (1965).
Doering, W., von, E. & Wiley, D. W. Heptafulvene (methylenecycloheptatriene). Tetrahedron 11, 183–198 (1960).
Hoffmann, R. & Woodward, R. B. Selection rules for concerted cycloaddition reactions. J. Am. Chem. Soc. 87, 2046–2048 (1965).
Hoffmann, R. & Woodward, R. B. Orbital symmetries and endo–exo relationships in concerted cycloaddition reactions. J. Am. Chem. Soc. 87, 4388–4389 (1965).
Cookson, R. C., Drake, B. V., Hudec, J. & Morrison, A . The adduct of tropone and cyclopentadiene: a new type of cyclic reaction. Chem. Commun. 15–16 (1966).
Takeshita, H., Wada, Y., Mori, A. & Hatsui, T . The cycloaddition reaction of isobenzofuran with some tropones. Chem. Lett. 2, 335–336 (1973).
Dahnke, K. R. & Paquette, L. A. Exploratory synthetic studies involving the tricyclo[9.3.0.02,8]tetradecane ring system peculiar to the cyathins. J. Org. Chem. 59, 885–899 (1994).
Li, P. & Yamamoto, H. Formal synthesis of platencin. Chem. Commun. 46, 6294–6295 (2010).
Li, P. & Yamamoto, H. Lewis acid catalyzed inverse-electron-demand Diels–Alder reaction of tropones. J. Am. Chem. Soc. 131, 16628–16629 (2009).
Houk, K. N., Luskus, L. J. & Bhacca, N. S. Novel double [6+4] cycloaddition of tropone to dimethylfulvene. J. Am. Chem. Soc. 92, 6392–6394 (1970).
Trost, B. M. & Seoane, P. R. [6+3] Cycloaddition to nine-membered ring carbocycles. J. Am. Chem. Soc. 109, 615–617 (1987).
Trost, B. M., McDougall, P. J., Hartmann, O. & Wathen, P. T. Asymmetric synthesis of bicyclo[4.3.1]decadienes and bicyclo[3.3.2]decadienes via [6+3] trimethylenemethane cycloaddition with tropones. J. Am. Chem. Soc. 130, 14960–14961 (2008).
Trost, B. M. & McDougall, P. J. Access to a welwitindolinone core using sequential cycloadditions. Org. Lett. 11, 3782–3785 (2009).
Rigby, J. H. & Fleming, M. Construction of the ingenane core using an Fe(III) or Ti(IV) Lewis acid-catalyzed intramolecular [6+4] cycloaddition. Tetrahedron Lett. 43, 8643–8646 (2002).
Isakovic, L., Ashenhurst, J. A. & Gleason, J. L. Application of Lewis acid catalyzed tropone [6+4] cycloadditions to the synthesis of the core of CP-225,917. Org. Lett. 3, 4189–4192 (2001).
Ashenhurst, J. A. & Gleason, J. L. CP-225,917 synthetic studies: unusual hydroboration regioselectivity influenced by remote functional groups. Tetrahedron Lett. 49, 504–507 (2008).
Ashenhurst, J. A., Isakovic, L. & Gleason, J. L. Application of a [6+4] cycloaddition strategy toward the total synthesis of CP-225,917. Tetrahedron 66, 368–378 (2010).
Nozoe, T., Mukai, T., Osaka, K. & Shishido, N. Synthesis of some 8,8-disubstituted heptafulvene derivatives. Bull. Chem. Soc. Jpn 34, 1384–1390 (1961).
Mukai, T., Tezuka, T. & Akasaki, Y. Tropone dimer. A new type of photocycloaddition reaction. J. Am. Chem. Soc. 88, 5025–5026 (1966).
Cantrell, T. S. Photochemical 8+2 cycloadditions of tropone. J. Am. Chem. Soc. 93, 2540–2541 (1971).
Rigby, J. H. et al. Metal-promoted higher-order cycloaddition reactions. Stereochemical, regiochemical, and mechanistic aspects of the [6π+4π] reaction. J. Am. Chem. Soc. 115, 1382–1396 (1993).
Xie, M. et al. Catalytic asymmetric [8+2] cycloaddition: synthesis of cycloheptatriene-fused pyrrole derivatives. Angew. Chem. Int. Ed. 52, 5604–5607 (2013).
Firrell, N. F. & Hickmott, P. W . Enamine chemistry. Part VI. Structure and proton magnetic resonance spectra of dienamines. J. Chem. Soc. B 293–298 (1969).
Firrell, N. F. & Hickmott, P. W . Enamine chemistry. Part VII. Synthesis and structure of dienamines of 3-alkyl-5,5-dimethylcyclohex-2-enones. Factors affecting the formation and relative stability of exo- and endo-cyclic diene systems. J. Chem. Soc. C 716–719 (1970).
Hayashi, R., Feltenberger, J. B. & Hsung, R. P. Torquoselective ring closures of chiral amido trienes derived from allenamides. A tandem allene isomerization−pericyclic ring-closure−intramolecular Diels−Alder cycloaddition. Org. Lett. 12, 1152–1155 (2010).
Xia, A.-B., Xu, D.-Q., Wu, C., Zhao, L. & Xu, Z.-Y. Organocatalytic Diels–Alder reactions catalysed by supramolecular self-assemblies formed from chiral amines and poly(alkene glycol)s. Chem. Eur. J. 18, 1055–1059 (2012).
Sundén, H., Ibrahem, I., Eriksson, L. & Córdova, A. Direct catalytic enantioselective aza-Diels–Alder reactions. Angew. Chem. Int. Ed. 44, 4877–4880 (2005).
Xu, D.-Q. et al. In situ enamine activation in aqueous salt solutions: highly efficient asymmetric organocatalytic Diels–Alder reaction of cyclohexenones with nitroolefins. Angew. Chem. Int. Ed. 48, 3821–3824 (2009).
Feng, X. et al. Stereodivergence in amine-catalyzed regioselective [4+2] cycloadditions of β-substituted cyclic enones and polyconjugated malononitriles. J. Am. Chem. Soc. 134, 19942–19947 (2012).
Bencivenni, G., Galzerano, P., Mazzanti, A., Bartoli, G. & Melchiorre, P. Direct asymmetric vinylogous Michael addition of cyclic enones to nitroalkenes via dienamine catalysis. Proc. Natl Acad. Sci. 107, 20642–20647 (2010).
Bastida, D., Liu, Y., Tian, X., Escudero-Adán, E. & Melchiorre, P. Asymmetric vinylogous aldol reaction via H-bond-directing dienamine catalysis. Org. Lett. 15, 220–223 (2013).
Bencivenni, G. et al. Targeting structural and stereochemical complexity by organocascade catalysis: construction of spirocyclic oxindoles having multiple stereocenters. Angew. Chem. Int. Ed. 48, 7200–7203 (2009).
Gu, X. et al. Direct catalytic asymmetric doubly vinylogous Michael addition of α,β-unsaturated γ-butyrolactams to dienones. Angew. Chem. Int. Ed. 54, 10249–10253 (2015).
Pellissier, H. Asymmetric organocatalytic cycloadditions. Tetrahedron 68, 2197–2232 (2012).
Mose, R., Jensen, M. E., Preegel, G. & Jørgensen, K. A. Direct access to multifunctionalized norcamphor scaffolds by asymmetric organocatalytic Diels–Alder reactions. Angew. Chem. Int. Ed. 54, 13630–13634 (2015).
Melchiorre, P. Cinchona-based primary amine catalysis in the asymmetric functionalization of carbonyl compounds. Angew. Chem. Int. Ed. 51, 9748–9770 (2012).
Oda, M., Tani, H. & Kitahara, Y. Reversible additions of an enamine to 8,8-disubstituted heptafulvenes. J. Chem. Soc. D http://dx.doi.org/10.1039/C2969000739A (1969).
Kitahara, Y. & Oda, M. The Jerusalem Symposia on Quantum Chemistry and Biochemistry (eds Bergmann, E.D. & Pullman, B.) 284–295 (The Jerusalem Academy of Sciences and Humanities, 1971).
Mori, M., Hayamizu, A. & Kanematsu, K. Frontier-controlled cycloaddition reactions of cyclopentadienones having electron-donating or -attracting substituents: configuration of adducts and kinetic studies. J. Chem. Soc. Perkin Trans. 1259–1272 (1981).
Oda, M., Funamizu, M. & Kitahara, Y. Cycloaddition reactions of tropone with enamines. J. Chem. Soc. D 737–738 (1969).
Lam, Y.-H. & Houk, K. N. Origins of stereoselectivity in intramolecular aldol reactions catalyzed by cinchona amines. J. Am. Chem. Soc. 137, 2116–2127 (2015).
Simon, A., Lam, Y.-H. & Houk, K. N. Transition states of vicinal diamine-catalyzed aldol reactions. J. Am. Chem. Soc. 138, 503–506 (2016).
Acknowledgements
This work was made possible by grants from Aarhus University and Carlsberg Foundation's ‘Semper Ardens’ programme. This research was supported by European Social Fund's Doctoral Studies and Internationalisation Programme DoRa, which is carried out by Foundation Archimedes. Our thanks are expressed to L. Næsborg, V. H. Lauritsen and M. E. Jensen for X-ray analysis.
Author information
Authors and Affiliations
Contributions
R.M. and G.P. optimized the reactions. R.M., G.P., J.L. and E.H.I. performed the experiments. S.J. performed the calculations. R.M. and K.A.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5205 kb)
Supplementary information
Crystallographic data for compound 4aA (CIF 182 kb)
Supplementary information
Crystallographic data for compound 11 (CIF 30 kb)
Supplementary information
Structure factors file for compound 11 (HKL 767 kb)
Supplementary information
Crystallographic data for compound 15 (CIF 1534 kb)
Supplementary information
Crystallographic data for compound 16 (CIF 15 kb)
Supplementary information
Structure factors file for compound 16 (HKL 717 kb)
Rights and permissions
About this article
Cite this article
Mose, R., Preegel, G., Larsen, J. et al. Organocatalytic stereoselective [8+2] and [6+4] cycloadditions. Nature Chem 9, 487–492 (2017). https://doi.org/10.1038/nchem.2682
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2682
This article is cited by
-
Enantioselective organocatalytic cycloadditions for the synthesis of medium-sized rings
Nature Synthesis (2023)
-
Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Nature Chemistry (2020)
-
N-Heterocyclic carbene-catalyzed enantioselective hetero-[10 + 2] annulation
Communications Chemistry (2020)
-
Enzyme-catalysed [6+4] cycloadditions in the biosynthesis of natural products
Nature (2019)