Abstract
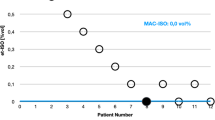
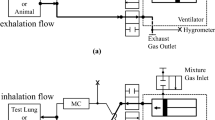
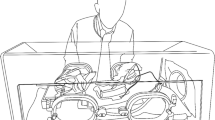
Anesthetic induction chambers used for medical research are a substantial source of waste anesthetic gas (WAG). Ideally, any generated waste gas should be actively vented away from personnel operating the chamber by either a ventilated hood or snorkel. Unfortunately, the ideal environment for anesthetizing rodents is not always available. In an effort to create a safer environment, the authors designed a system to reduce WAG. This system is portable, can be adapted to different precision vaporizing anesthetic systems and fits in a variety of physical locations. The system flushes anesthetic gas out of an induction chamber before operators open the chamber. To ensure that the system was adequately flushing the anesthetic gas, the authors measured WAG concentration in the environment above the induction chamber and directly behind the vent of an activated charcoal filter. They also compared the efficiency of the filters in vertical and horizontal positions. Finally, they measured the recovery time for mice and rats after flushing the anesthetic gas from an induction chamber. The results show that flushing the induction chamber was an inexpensive and effective method for reducing WAG accumulation in the air surrounding the chamber.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mazze, R.I., Wilson, A.I., Rice, S.A. & Baden, J.M. Fetal development in mice exposed to isoflurane. Teratology 32, 339–345 (1985).
Kober, F., Iltis, I., Cozzone, P.J. & Bernard, M. Cine-MRI assessment of cardiac function in mice anesthetized with ketamine/xylazine and isoflurane. MAGMA 17, 157–161 (2004).
Szczesny, G., Veihelmann, A., Massberg, S., Nolte, D. & Messmer, K. Long-term anaesthesia using inhalatory isoflurane in different strains of mice—the haemodynamic effects. Lab. Anim. 38, 64–69 (2004).
Smith, J.C. & Bolon, B. Atmospheric waste isoflurane concentrations using conventional equipment and rat anesthesia protocols. Contemp. Top. Lab. Anim. Sci. 41, 10–17 (2002).
Hoerauf, K.H., Koller, C., Jakob, W., Taeger, K. & Hobbhahn, J. Isoflurane waste gas exposure during general anaesthesia: the laryngeal mask compared with tracheal intubation. Br. J. Anaesth. 77, 189–193 (1996).
National Institute for Occupational Safety and Health. Criteria for a Recommended Standard: Occupational Exposure to Waste Anesthetic Gases and Vapors Publication No 77-140. (National Institute for Occupational Safety and Health, Cincinnati, OH, 1977).
Centers for Disease Control and Prevention. Request for information on waste halogenated anesthetic agents: isoflurane, desflurane, and sevoflurane. Fed. Regist. 71, 8859–8860 (2006).
Fish, R.E., Brown, M.J., Danneman, P.J. & Karas, A.Z. (eds.) Anesthesia and Analgesia in Laboratory Animals 2nd edn (Academic, London, 2008).
Cooper, D., Errede, D. & Streifel, A. Assessment of occupational exposure to isoflurane administered in an anesthetic chamber within a horizontal laminar flow clean bench. Contemp. Top. Lab. Anim. Sci. 37, 64–67 (1998).
Smith, J.A. Anesthetic pollution and waste anesthetic gas scavenging. Semin. Vet. Med. Surg. 8, 90–103 (1993).
American College of Veterinary Anesthesiologists ad hoc Committee on Waste Anesthetic Gas Pollution and Its Control. Commentary and recommendations on control of waste anesthetic gases in the workplace. J. Am. Vet. Med. Assoc. 209, 75–77 (1996).
Task Force on Trace Anesthetic Gases. Waste Anesthetic Gases: Information for Management in Anesthetizing Areas and the Postanesthesia Care Unit (PACU) (American Society of Anesthesiologists, Park Ridge, IL, 1999).
Meyer, R.E. Anesthesia hazards to animal workers. Occ. Med. 14, 225–234 (1999).
Smith, J.C. & Bolon, B. Isoflurane leakage from non-rebreathing rodent anesthesia circuits: comparison of emissions from conventional and modified ports. Lab. Anim. 40, 200–209 (2006).
Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996).
Van Dort, C.J., Baghdoyan, H.A. & Lydic, R. Adenosine A1 and A2A receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J. Neurosci. 29, 871–881 (2009).
Vanini, G., Watson, C.J., Lydic, R. & Baghdoyan, H.A. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology 109, 978–988 (2008).
Taylor, D.K. & Mook, D.M. Isoflurane waste anesthetic gas concentrations associated with the open-drop method. J. Am. Assoc. Lab. Anim. Sci. 48, 61–64 (2009).
Hunt, K.H. Resistance in respiratory valves and canisters. Anesthesiology 16, 190–205 (1955).
Smith, J.C. & Bolon, B. Comparison of three commercially available activated charcoal canisters for passive scavenging of waste isoflurane during conventional rodent anesthesia. Contemp. Top. Lab. Anim. Sci. 42, 10–15 (2003).
Nunn, J.N. Nunn's Applied Respiratory Physiology 4th edn. 583–593 (Butterworth-Hinemann, Oxford, 1993).
Acknowledgements
J.W. and M.C.D. conceived, designed and carried out the experiments; analyzed the data; contributed reagents, materials and analysis tools; and wrote the paper. We thank Toby Donajkowski for fabrication of the induction chamber and Steven F. Bolling, PhD, and Mitchell Seymour, MS, for providing animals. We thank David Marlow of the University of Michigan's Biomedical Technology Services and Ben Phillips for technical support. We thank John A. Williams, the Director of the Department of Molecular and Integrative Physiology, and Joseph M. Metzger, the Director of the Center for Integrative Genomics, for providing necessary facilities and financial support.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wolforth, J., Dyson, M. Flushing induction chambers used for rodent anesthesia to reduce waste anesthetic gas. Lab Anim 40, 76–83 (2011). https://doi.org/10.1038/laban0311-76
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/laban0311-76