According to the Pan American Health Organization, 7 out of 10 deaths in people over 70 are from non-communicable diseases (NCDs). Aging itself is the single greatest risk factor for the majority of these conditions. In 2016, the Milken Institute reported that NCDs cost the US government $1.1 trillion (£0.9 trillion). This is 6% of the US’ total gross domestic product (GDP). Increasing healthy life expectancy by just one year in the US could be worth $38 trillion (£31 trillion).
Founded in 2015, BioViva Science aims to leverage gene therapy to prevent, treat, or reverse the processes underpinning the most common diseases in the developed world: aging-associated NCDs.
“Almost every disease is affected by aging, and this has huge economic, psychological, and healthcare implications. A focus on aging means that we can target a broad range of diseases,” said George Church, scientific advisor to BioViva, entrepreneur, and professor at Harvard Medical School and the Massachusetts Institute of Technology (MIT).
Lengthening healthy human lifespans
BioViva uses combinatorial gene therapies to target the underpinning causes of aging.
“We develop gene therapies to help you live longer and better,” said Elizabeth Parrish, founder and CEO of BioViva. “There are a large number of unmet therapeutic needs in this area.”
Building a pipeline
BioViva’s journey in gene therapy began with its use of an adeno-associated virus (AAV) vector. The company’s lead gene therapy, BV-702, is AAV-based and is in preclinical development for Alzheimer’s disease. It improved cognition and calculated biological age (telomere length) in a small non-controlled investigator-sponsored study (n=5).
The company has a patent for BV-130, another AAV therapeutic, which has shown early benefits in immune senescence in a case study (n=1).
BioViva is building a gene-therapy platform using a cytomegalovirus (CMV) that can carry a genetic payload three times larger than the AAV. The company hopes to expand this to at least 10 times the AAV payload size. CMV can be injected or delivered intranasally. Its established safety profile and lower immunogenicity make it redosable in animal models (Fig. 1). CMV does not integrate, which could reduce off-target effects.
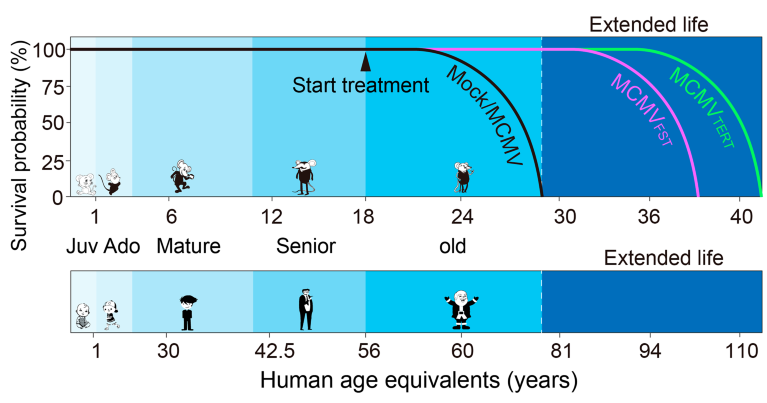
Fig. 1 | The survival curve of mice in each group was determined by a Kaplan–Meier survival curve. χ2 test, p<0.001 TERT-IP vs. WT-IP and TERT-IN vs. WT-IN group at the 50% survival probability; p<0.001 FST-IP vs. WT-IP and FST-IN vs. WT-IN group at the 50% survival probability. n=8 per group. C57BL/6J mice and human age equivalence at the start of experimental treatment. MCMV, mouse cytomegalovirus; FST, follistatin; TERT, telomerase reverse transcriptase.
“Gene therapy for aging-related diseases needs efficient delivery vectors. The greater payload of the CMV vector allows delivery of larger or multiple genes, which will be important in the treatment of complex disorders,” said Church.
BioViva’s early-stage CMV projects are looking at metabolic disease, frailty, cardiovascular disease, chronic kidney disease (CKD), and chronic obstructive pulmonary disease (COPD).
“We are applying for two pre-investigational new drugs (INDs): one for our intranasal gene therapy for Alzheimer’s disease and another to look at age-related metabolic dysfunction with secondary endpoints in age-related muscle loss. These pre-IND meetings will set the path for our regulatory framework,” said Parrish.
Once the gene therapies have reached proof-of-concept studies in humans, Parrish sees many possibilities.
“We would like to see our gene therapies reach the market, and we are flexible as to how this happens. We are open to licensing, partnerships, and joint ventures,” said Parrish.
Building the company through investment and partnership
BioViva is currently a privately held company. It is seeking to raise $27.5 million in a Series A round. The company previously raised $5 million in seed funding. The Series A will be used to fund BV-702 to phase 1b, set up pre-IND studies for a new CMV gene therapy, and continue CMV research.
BioViva hopes to capture a share of the $325 billion regenerative-medicine market and the trillion-plus aging market. The large size of the age-related disease market means BioViva’s gene therapies are expected to be more affordable than those for rare diseases.