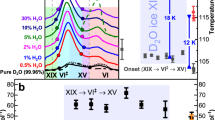
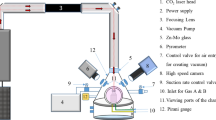
Abstract
When a piece of ice Ih whose grains are not too small is irradiated by infrared or radio-frequency light, internal melt figures are often formed. They are often called Tyndall figures or Tyndall flowers after John Tyndall, who was the first to understand them1. No other substance has been reported to form internal melt figures, although any substance that melts to a denser liquid should form them at the proper conditions. The shape of the figures will, of course, depend on the symmetry of the crystal. Tetrahydrofuran clathrate hydrate2, which has the nominal composition C4H8O.17H2O, melts to a denser homogeneous liquid at 4.1 °C with a fractional change of volume of −0.0147±0.00033. Internal melt figures should therefore form inside the clathrate if it is heated internally. From the symmetry and the structure of the crystal, the figures should be octahedra2. This letter describes this second type of internal melt figure, which was discovered 130 years after the first, and suggests that melt figures should occur in many other crystals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tyndall, J. Proc. R. Soc. A. 9, 76–80 (1858).
Davidson, D. W. in Water a Comprehensive Treatise Vol. 2 (ed. Franks, F.) 115–234 (Plenum, New York, 1973).
Gough, S. R. & Davidson, D. W. Can. J. Chem. 49, 2691–2699 (1971).
Kass, M. & Magun, S. Z. Krist. 116, 354–370 (1961).
Hawkins, R. E. & Davidson, D. W. J. phys. Chem. 70, 1889–1894 (1966).
Wickert, J. N., Tamplin, W. S. & Shank, R. L. Chem. Engng Progr. Symp. Ser. 2 48, 92–96 (1952).
Tamman, G. & Krige, G. R. J. Z. anorg. allgem. Chem. 146, 179–195 (1925).
Müller-Krumthar, H. in Physics of Ice (eds Riel, N., Bullemer, B. & Engelhardt, H.) 132 (Plenum, New York, 1969).
Adams, J. M. & Lewis, W. Rev. Sci. Instrum. 5, 400–402 (1934).
Knight, C. A. & Knight, N. C. Science 150, 1819–1821 (1965).
Hobbs, P. V. Ice Physics Ch. 6. (Clarendon, Oxford, 1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McLaurin, G., Whalley, E. Negative octahedral snowflakes or Tyndall figures in tetrahydrofuran clathrate hydrate. Nature 332, 711–712 (1988). https://doi.org/10.1038/332711a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/332711a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.