Abstract
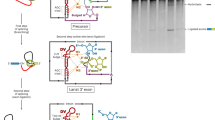
Analysis of messenger RNA splicing in yeast and in metazoa has led to the identification of an RNA molecule in a lariat conformation. This structure has been found as an mRNA splicing intermediate in vitro1,2 and identical molecules have been identified in vivo3–5. Lariat formation involves cleavage of the precursor at the 5′ splice site (5′ SS) and the formation of a 2′–5′ phosphodiester bond between the guanosine residue at the 5′ end of the intron and an adenosine within the intron1,2. The yeast branchpoint is located within the absolutely conserved TACTAAC box (that is, the last A of the TACTAAC box is the site of formation of the 2′–5′ phosphodiester bond with the 5′ end of the intron)3,4. Moreover, efficient 5′ SS cleavage and lariat formation require proper sequences at the 5′ splice junction and within the TACTAAC box6–12. Here we demonstrate that 5′ SS cleavage and lariat formation take place in vitro in the absence of the 3′ SS and much of the 3′ junction. These results are discussed in light of possible differences between yeast and metazoan mRNA splicing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ruskin, B., Krainer, A. R., Maniatis, T. & Green, M. R. Cell 38, 317–331 (1984).
Padgett, R. A., Konarska, M., Grabowski, P. J., Hardy, S. F. & Sharp, P. A. Science 225, 898–903 (1984).
Rodriguez, J. R., Pikielny, C. W. & Rosbash, M. Cell 39, 603–610 (1984).
Domdey, H. et al. Cell 39, 611–621 (1984).
Zeitlin, S. & Efstratiadis, A. Cell 39, 589–604 (1984).
Gallwitz, D. Proc. natn. Acad. Sci. U.S.A. 79, 3493–3497 (1982).
Pikielney, C. W., Teem, J. L. & Rosbash, M. Cell 34, 395–403 (1983).
Langford, C. J. & Gallwitz, D. Cell 33, 519–527 (1983).
Langford, C. J., Klinz, F. J., Donath, C. & Gallwitz, D. Cell 36, 645–653 (1984).
Parker, R. & Guthrie, C. Cell 41, 107–118 (1985).
Newman, A. J., Lin, R., Cheng, S. C. & Abelson, J. Cell 42, 335–344 (1985).
Pikielny, C. W. & Rosbash, M. Cell 41, 119–126 (1985).
Lin, R.-J., Newman, A. J., Cheng, S.-C. & Abelson, J. J. biol. Chem. (in the press).
Brody, E. & Abelson, J. Science 228, 963–967 (1985).
Ruskin, B., Pikielny, C. W., Rosbash, M. & Green, M. R. Proc. natn. Acad. Sci. U.S.A. (in the press).
Ruskin, B. & Green, M. R. Science 229, 135–140 (1985).
Reed, R. & Maniatis, T. Cell 41, 95–105 (1985).
Ruskin, B. & Green, M. R. Nature (this issue).
Konarska, M. M., Grabowski, P. J., Padgett, R. A. & Sharp, P. A. Nature 313, 552–557 (1985).
Ruskin, B., Greene, J. M. & Green, M. R. Cell 41, 833–844 (1985).
Rautmann, G. & Breathnach, R. Nature 315, 430–432 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rymond, B., Rosbash, M. Cleavage of 5′ splice site and lariat formation are independent of 3′ splice site in yeast mRNA splicing. Nature 317, 735–737 (1985). https://doi.org/10.1038/317735a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/317735a0
This article is cited by
-
Single Molecule Cluster Analysis dissects splicing pathway conformational dynamics
Nature Methods (2015)
-
Exon ligation is proofread by the DExD/H-box ATPase Prp22p
Nature Structural & Molecular Biology (2006)
-
Scanning from an independently specified branch point defines the 3′ splice site of mammalian introns
Nature (1989)
-
The role of small nuclear ribonucleoprotein particles in pre-mRNA splicing
Nature (1987)
-
Electrophoresis of ribonucleoproteins reveals an ordered assembly pathway of yeast splicing complexes
Nature (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.