Abstract
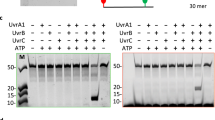
Treatment of Escherichia coli with radiation or chemical carcinogens induces a number of cellular functions to counteract the damage inflicted by such treatments (see ref. 1 for a review). One of these inducible functions is the SOS response, which is controlled by the recA and lexA genes: single-stranded DNA produced as a result of DNA damage binds to recA protein (RecA), activating its protease function; activated RecA cuts lexA protein (LexA), the represser of the SOS genes, thereby inactivating it and turning on these genes2–4. The SOS genes include recA and lexA themselves, and recent work has shown that the two genes have similar operators where LexA binds in vitro, inhibiting their transcription3,4. It is anticipated that other genes under the control of recA and lexA will have similar operators. Recent genetic5,6 and biochemical7 data suggest that the excision repair genes uvrA and uvrB are part of the recA–lexA regulon and are at least partly responsible for recA–lexA-mediated inducible DNA repair. In support of these results we have reported that uvrB has a promoter that is repressed by LexA in vitro8. We now show that uvrA has an operator similar to those of other SOS genes and that LexA binds to this operator and inhibits the transcription of the uvrA gene in vitro.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Howard-Flanders, P. Scient. Am. 245, 72–80 (1981).
Little, J. W., Edmiston, S. H., Pacelli, L. Z. & Mount, D. W. Proc. natn. Acad. Sci. U.S.A. 77, 3225–3229 (1980).
Little, J. W., Mount, D. W. & Yanisch-Perron, C. R. Proc. natn. Acad. Sci. U.S.A. 78, 4199–4203 (1981).
Brent, R. & Ptashne, M. Proc. natn. Acad. Sci. U.S.A. 78, 4204–4281 (1981).
Kenyon, C. J. & Walker, G. C. Nature 289, 808–810 (1981).
Fogliano, M. & Schendel, P. Nature 289, 196–198 (1981).
Kacinski, B. M., Sancar, A. & Rupp, W. D. Nucleic Acids Res. 9, 4495–4508 (1981).
Sancar, G. B., Sancar, A., Little, J. W. & Rupp, W. D. Cell 28, 523–530 (1982).
Sancar, A., Williams, K. R., Chase, J. W. & Rupp, W. D. Proc. natn. Acad. Sci. U.S.A. 78, 4274–4278 (1981).
Sancar, A. et al. J. molec. Biol. 148, 45–62 (1981).
Ebina, Y. et al. Gene 15, 119–126 (1981).
Miller, H. I., Kirk, M. & Echols, H. Proc. natn. Acad. Sci. U.S.A. 78, 6754–6758 (1980).
Levine, A. & Rupp, W. D. in Microbiology-1978 (ed. Schlesinger, D.) 163–166 (American Society for Microbiology, Washington DC, 1978).
Maxam, A. & Gilbert, W. Meth. Enzym. 65, 499–560 (1980).
Galas, D. & Schmitz, A. Nucleic Acids Res. 5, 3157–3170 (1978).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sancar, A., Sancar, G., Rupp, W. et al. LexA protein inhibits transcription of the E. coli uvrA gene in vitro. Nature 298, 96–98 (1982). https://doi.org/10.1038/298096a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/298096a0
This article is cited by
-
When push comes to shove - RNA polymerase and DNA-bound protein roadblocks
Biophysical Reviews (2023)
-
The Nobel Prize in Chemistry 2015: Exciting discoveries in DNA repair by Aziz Sancar
Science China Life Sciences (2016)
-
Changes in ffh, uvrA, groES and dnaK mRNA Abundance as a Function of Acid-Adaptation and Growth Phase in Bifidobacterium longum BBMN68 Isolated from Healthy Centenarians
Current Microbiology (2011)
-
Analysis of the regulatory elements of the Escherichia coli uvrC gene by construction of operon fusions
Molecular and General Genetics MGG (1988)
-
Induction of phr gene expression by irradiation of ultraviolet light in Escherichia coli
Molecular and General Genetics MGG (1987)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.