Abstract
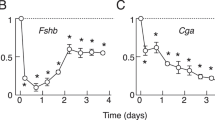
Calcium (Ca2+) seems to have an informational role in many tissues. In particular, it fulfills the requirements of a second messenger for gonadotropin releasing hormone (GnRH)-stim-ulated luteinizing hormone (LH) release from the pituitary gonadotrope (see ref. 1 for review). Very little is known about the effect of this ion on intracellular targets or the mechanism by which Ca2+ mobilization stimulates LH release. One intracellular target for Ca2+ is calmodulin, a ubiquitous intracellular Ca2+ receptor that has been shown to modulate many cellular functions, including cyclic nucleotide and glycogen metabolism, protein phosphorylation, microtubule assembly and disassembly, Ca2+ flux, and the activities of NAD kinase, tryptophan 5′ monooxidase and phospholipase A2 (see refs 2–5 for reviews). We have now used a specific and sensitive radioimmunoassay to determine the quantity and distribution of calmodulin in the gonadotrope before and during GnRH-stimulated LH release. The data indicate that GnRH stimulates redistribution of calmodulin from the cytosol to the plasma membrane and suggest that the molecule may have a role in the mechanism of stimulus–secretion coupling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Conn, P. M. et al. Endocr. Rev. 2, 174–185 (1981).
Cheung, W. Y. Science 207, 19–27 (1980).
Klee, C. B., Crouch, T. H. & Richman, P. G. A. Rev. Biochem. 49, 487–516 (1980).
Means, A. R. & Dedman, J. R. Nature 285, 73–77 (1980).
Wang, J. H. & Waisman, D. M. Curr. Topics cell. Regulation 15, 47–105 (1979).
Marian, J., Cooper, R. L. & Conn, P. M. Molec. Pharmac. 19, 399–405 (1981).
Chafouleas, J. G., Conn, P. M., Dedman, J. R. & Means, A. R. Endocrinology A106, 289 (1980).
Larsen, F. L. & Vincenzi, F. F. Science 204, 306–308 (1979).
Pershadsingh, H. A., McDaniel, M. L., Bry, C. G., Lacy, P. E. & McDonald, J. M. Nature 288, 492–495 (1980).
Pershadsingh, H. A. & McDonald, J. M. Nature 281, 495–497 (1980).
deLorenzo, R. J., Freedman, S. D., Yohe, W. B. & Maurer, S. C. Proc. natn. Acad. Sci. U.S.A. 76, 1838–1842 (1979).
Grab, D. J., Berzins, K., Cohen, R. S. & Siekevitz, P. J. J. biol. Chem. 254, 8690–8696 (1979).
Ilundian, A. & Naftalin, R. J. Nature 279, 446–468 (1979).
Conn, P. M., Smith, R. G. & Rogers, D. J. biol Chem. 256, 1098–1100 (1981).
Hazum, E., Cuatrecasas, P., Marian, J. & Conn, P. M. Proc. natn. Acad. Sci. U.S.A. 77, 6692–6695 (1980).
Salisbury, J. L., Condeelis, J. S. & Satir, P. J. Cell Biol. 87, 132–141 (1980).
Linden, C. D., Dedman, J. R., Chafouleas, J. G., Means, A. R. & Roth, T. F. Proc. natn. Acad. Sci. U.S.A. 78, 308–312 (1981).
Suzuki, K. & Kono, T. J. Biol. Chem. 254, 9786–9794 (1979).
Suzuki, K. & Kono, T. Proc. natn. Acad. Sci. U.S.A. 77, 2542–2545 (1980).
Cushman, S. W. & Wardzala, L. J. J. biol. Chem. 255, 4758–4762 (1980).
Wray, W., Conn, P. M. & Wray, V. P. in Meth. Cell Biol. 14, 69–86 (1977).
Schneider, W. in Selected Data for Molecular Biology and Biochemistry, K3–K14 (Chemical Rubber Press, 1970).
Chafouleas, J. G., Dedman, J. R., Munjaal, R. P. & Means, A. R. J. biol. Chem. 254, 10262–10267 (1979).
Conn, P. M., Rogers, D. C. & Sheffield, T. Endocrinol. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Conn, P., Chafouleas, J., Rogers, D. et al. Gonadotropin releasing hormone stimulates calmodulin redistribution in rat pituitary. Nature 292, 264–265 (1981). https://doi.org/10.1038/292264a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/292264a0
This article is cited by
-
Gonadotropin releasing hormone stimulation of pituitary cell 3′–5′ cyclic AMP in a carp(Cyprinus carpio) is dependent on extracellular calcium
Journal of Biosciences (1994)
-
Calmodulin loss in vascular smooth muscle following Triton X-100 or saponin skinning
Pfl�gers Archiv European Journal of Physiology (1989)
-
Distribution of calmodulin and calmodulin-binding proteins in bovine pituitary: association of myosin light chain kinase with pituitary secretory granule membranes
Molecular and Cellular Biochemistry (1987)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.