Abstract
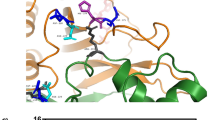
D-2,3-DIPHOSPHOGLYCERATE (DPG) facilitates the transfer of oxygen from human red blood cells to the tissues by lowering the oxygen affinity of haemoglobin1. It does so by combining preferentially with one of the two alternative forms of haemoglobin, namely the deoxy form, in the ratio of 1 mol per mol tetramer. Its binding site was predicted from biochemical and model building experiments and then determined directly by X-ray crystallography2. The site lies at the entrance to the central cavity between the N-termini of the β chains and is surrounded by four pairs of basic groups: the α amino group of valine 1 and the side chains of histidines 2 and 143, and of lysine 82. The basic groups are related in pairs by the molecular dyad and arranged so as to complement the acidic groups of DPG by forming seven salt bridges (Fig. 1). On oxygenation the N-termini of the β chains move apart and the cavity closes up so that the stereochemical complementarity is lost3. Oxyhaemoglobin does also bind DPG, but much more weakly, and the binding site is still unknown.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Benesch, R., and Benesch, R. E., Nature, 221, 618 (1969).
Arnone, A., Nature, 237, 146 (1972).
Perutz, M. F., Nature, 228, 734 (1970).
Rapoport, S., and Guest, G. M., J. biol. Chem., 138, 269 (1941).
Johnson, L. F., and Tate, M. E., Can. J. Chem., 47, 63 (1969).
Perutz, M. F., J. Cryst. Growth, 2, 54 (1968).
Muirhead, H., and Greer, J., Nature, 228, 516 (1970).
Blank, G. E., Pletcher, J., and Sax, M., Biochem. biophys. Res. Commun., 44, 319 (1971).
Richards, F. M., J. molec. Biol., 37, 225 (1968).
Kilmartin, J. V., Biochem. J., 133, 725 (1973).
Matsuda, G., Maita, T., Mizuno, K., and Ota, H., Nature new Biol., 244, 244 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
ARNONE, A., PERUTZ, M. Structure of inositol hexaphosphate–human deoxyhaemoglobin complex. Nature 249, 34–36 (1974). https://doi.org/10.1038/249034a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/249034a0
This article is cited by
-
Origin of complexity in haemoglobin evolution
Nature (2020)
-
Oxygen dissociation from ferrous oxygenated human hemoglobin:haptoglobin complexes confirms that in the R-state α and β chains are functionally heterogeneous
Scientific Reports (2019)
-
Phosphorylation of Leghemoglobin at S45 is Most Effective to Disrupt the Molecular Environment of Its Oxygen Binding Pocket
The Protein Journal (2015)
-
Quadrupole splitting temperature dependence of high and low affinity deoxyhemoglobin encapsulated in wet silica gel
Hyperfine Interactions (2005)
-
Engineered erythrocytes: influence of P50 rightward shift and oxemia on oxygen transport to tissues
Medical & Biological Engineering & Computing (1998)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.