Abstract
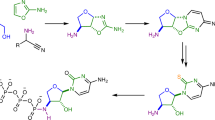
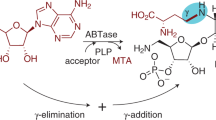
DURING the past few years the metabolism by mammalian cells of a number of natural and synthetic nucleosides structurally related to adenosine has been investigated in this1–5 and other laboratories6–10. In our studies with Ehrlich ascites cells, we have tried to establish a correlation between the structural variations and the utilization by cells of these nucleosides and have observed the following salient features: (a) In order to be phosphorylated to the 5′-triphosphate level, the adenosine analogue must have a primary amino group at the C-6 position of the purine moiety. Compounds that belong in this category are 3′-deoxyadenosine2,6, 3′-amino-3′-deoxyadenosine4 and 2-fluoroadenosine3. These three nucleosides were also found to be potent inhibitors of nucleic acid synthesis in Ehrlich ascites cells. Work in other laboratories has shown that 7-deazaadenosine7, xylosyladenine8, arabinosyladenine9 and the bases 2-azaadenine and 2,6-diaminopurine10 were also phosphorylated to 5′-triphosphate. 2,6-Diaminopurine is also metabolized to the triphosphate level by microbial cells11,12. Removal of the 6-amino group as in 3′-deoxynebularine resulted in complete absence of phosphorylation by ascites cells*4. (b) 6-Methylaminopurine ribonucleoside—an adenosine analogue with a 6-methyl-amino group—was metabolized to the monophosphate level only4. This was also found to be so for 6-methyl-aminopurine - 2′-deoxyribonucleoside, 6-methylaminopurine-3′-deoxyribonucleoside and 6-methylaminopurine5. (c) Replacement of the 6-amino group by 3′-deoxyadenosine with an ethylamino or dimethylamino group completely suppressed the capacity of the nucleoside for phosphorylation. This relationship was exemplified by 6-ethylaminopurine-3′-deoxyribonucleoside and 6-dimethylaminopurine-3′-deoxyribonucleoside4. Furthermore, 6-dimethylamino-3′-amino-3′-deoxyribonucleoside was also inactive, while its parent compound, as already mentioned, was readily phosphorylated. Nucleosides that were not phosphorylated had almost no effect on nucleic acid synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shigeura, H. T., and Boxer, G. E., Biochem. Biophys. Res. Commun., 17, 758 (1964).
Shigeura, H. T., and Gordon, C. N., J. Biol. Chem., 240, 806 (1965).
Shigeura, H. T., Boxer, G. E., Sampson, S. D., and Meloni, M. L., Arch. Biochem. Biophys., 111, 713 (1965).
Shigeura, H. T., Boxer, G. E., Meloni, M. L., and Sampson, S. D., Biochemistry, 5, 994 (1966).
Shigeura, H. T., Sampson, S. D., and Meloni, M. L., Arch. Biochem. Biophys., 115, 462 (1966).
Klenow, H., Biochim. Biophys. Acta, 76, 347 (1963).
Acs, G., Reich, E., and Mori, M., Proc. US Nat. Acad. Sci., 52, 493 (1964).
Ellis, D. B., and LePage, G. A., Mol. Pharmacol., 1, 231 (1965).
Brink, J. J., and LePage, G. A., Cancer Res., 24, 312 (1964).
Tatibana, M., and Yoshikawa, H., Biochim. Biophys. Acta, 57, 613 (1962).
Kornberg, A., and Pricer, jun., W. E., J. Biol. Chem., 193, 481 (1951).
Remy, C. N., and Smith, M. S., J. Biol. Chem., 228, 325 (1957).
Walton, E., Holly, F. W., and Nutt, R. F., Abstract No. 37c, Meeting of Amer. Chem. Soc., January 1965.
Robins, M. J., McCarthy, jun., J. R., and Robins, R. K., Biochemistry, 5, 224 (1966).
Magee, W. E., and Eberts, jun., F. S., Cancer Res., 21, 611 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SHIGEURA, H., SAMPSON, S. Structural Basis for Phosphorylation of Adenosine Congeners. Nature 215, 419–420 (1967). https://doi.org/10.1038/215419a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/215419a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.