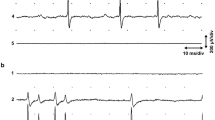
Abstract
THE active transport of sodium and potassium ions by sodium-rich extensor muscles of rat was much greater when these muscles were still connected to the central nervous system1 than when they were denervated during immersion in recovery fluid. Furthermore, while the sodium pump in denervated muscles was electrogenic, raising the membrane potential by as much as 12 mV above the potassium equilibrium potential during excretion of sodium ions2–4, the sodium–potassium pump was almost electrically neutral in innervated frog5 and rat6 muscles, suggesting a stimulation of uptake of potassium ions in the innervated preparation. In view of the depolarizing action of active uptake of potassium ions and of its possible relation to the generation of end-plate potentials, we examined the action of some cholinergic agents on active transport in sodium-rich extensor muscles. While acetylcholine was found to stimulate greatly the transport of sodium and potassium ions, eserine and decamethonium both appeared to inhibit active transport of these ions6. Eserine by inhibiting choline esterase activity7 and possibly by causing its release from nerve endings8 augments acetylcholine activity at the end plate. Decamethonium mimics the depolarizing action of acetylcholine at the end plate but cannot be broken down by choline esterase9. We considered it possible that these two substances might depolarize the membrane to the point where increased entrance of sodium ions, associated with the action potential, would mask any stimulating effect they might have on the active transport of potassium and sodium ions. We therefore placed four sets of sodium-rich extensor muscles in recovery fluid containing tetrodotoxin (10–7 g/ml.). This substance blocks the rapid inward movement of sodium ions, which produces the action potential in nerve and muscle10, but does not eliminate miniature end-plate potentials11,12. One set of muscles in each case was used as a control, while either eserine (50 µg/ml.) or decamethonium iodide (0.1 µg/ml.) was added to the recovery fluid containing their companion muscles. Both substances greatly stimulated the uptake of potassium ions in the presence of tetrodotoxin without having as significant an effect on active excretion of sodium ions. The muscles treated with eserine took up 16.5 m.equiv. more potassium ions, and excreted 6.7 m.equiv. more sodium ions/kg of muscle than their untreated companions. The difference in the case of potassium ions was very significant (P<0.01), but in the case of sodium ions was only slightly significant (0.05 < P < 0.1). Muscles treated with decamethonium during recovery contained 9.0 m.equiv. more potassium ions (P<0.01) on the average than their untreated companions. There was no significant difference in the excretion of sodium ions here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Kernan, R. P., J. Physiol., 179, 63P (1965).
Kernan, R. P., Nature, 193, 986 (1962).
Keynes, R. D., and Rybova, R., J. Physiol., 168, 58 (1963).
Mullins, L. J., and Awad, M. Z., J. Gen. Physiol., 48, 761 (1965).
Kernan, R. P., Nature, 210, 537 (1966).
Dockry, M., Kernan, R. P., and Tangney, A., J. Physiol., 186, 187 (1966).
Wilson, I. B., and Bergman, S., J. Biol. Chem., 186, 683 (1950).
Riker, jun., W. F., Werner, G., Roberts, J., and Kuperman, A., Ann. N.Y. Acad. Sci., 81, 328 (1959).
Zaimis, E., J. Physiol., 112, 176 (1951).
Narahashi, T., Moore, W., and Scott, W. R., J. Gen. Physiol., 47, 965 (1964).
Elmqvist, D., and Feldman, D. S., Acta Physiol. Scand., 64, 475 (1965).
Katz, B., and Miledi, R., J. Physiol., 185, 5 (1966).
Fatt, P., and Katz, B., J. Physiol., 118, 73 (1952).
Armstrong, W. McD., Amer. J. Physiol., 208, 61 (1965).
Steinbach, H. B., Proc. U.S. Nat. Acad. Sci., 38, 451 (1952).
Hurlbut, W. P., J. Gen. Physiol., 46, 1223 (1963).
Hubbard, J. I., and Løyning, Y., J. Physiol., 185, 205 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
KERNAN, R. Electrogenic Potassium Pump related to Generation of End-plate Potentials in Muscle. Nature 214, 725–726 (1967). https://doi.org/10.1038/214725a0
Issue Date:
DOI: https://doi.org/10.1038/214725a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.