Abstract
UNDER “steady state” conditions in the absence of its normal substrate (acetylcholine), or when the substrate concentration can be neglected, the inhibition of acetyl-cholinesterase (ChE) by insecticidal or related carbamates, such as 1-naphthyl N-methylcarbamate, o-isopropoxy-phenyl N-methylcarbamate (IPMC) and eserine, can be defined by the equation  The symbols v′ and v are the rates of substrate hydrolysis by the inhibited and uninhibited enzyme respectively, i is the concentration of carbamate, K1 the effective bimolecular carbamylation rate constant, and K2 the effective unimolecular rate constant for the hydrolytic regeneration of free enzyme1,2. It followed from (1) that if v′0/v0 represents the steady state inhibition obtaining in vivo or as a result of the pre-incubation of tissue and inhibitor in vitro, and v′z/vz that estimated after diluting the tissue z times and allowing a new steady state to be achieved
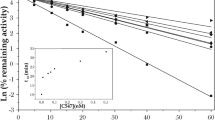
The symbols v′ and v are the rates of substrate hydrolysis by the inhibited and uninhibited enzyme respectively, i is the concentration of carbamate, K1 the effective bimolecular carbamylation rate constant, and K2 the effective unimolecular rate constant for the hydrolytic regeneration of free enzyme1,2. It followed from (1) that if v′0/v0 represents the steady state inhibition obtaining in vivo or as a result of the pre-incubation of tissue and inhibitor in vitro, and v′z/vz that estimated after diluting the tissue z times and allowing a new steady state to be achieved  When estimating human blood ChE inhibition as an index of accidental or occupational exposure to insecticidal carbamates it is therefore important to keep z minimal or to carry out the assay so rapidly that the change towards the new steady state can be neglected. Fortunately, the values of K2 for typical insecticidal carbamates2,3 are such that, if assays can be completed within a few minutes of sample dilution, the changes before and during the measurement are relatively small. A simple radiometric method for estimating blood ChE under field conditions has been described4 which enables an assay to be made within 2 min of dilution and involves diluting a 20 µl. blood sample a nominal eleven times. When this method was applied to samples of undiluted human blood pre-incubated with IPMC or eserine to simulate conditions in vivo, it was found that the new steady state effects of subsequent dilution were invariably less than those indicated by equation (2). The steady state inhibition obtaining at the instant of dilution was estimated by extrapolating the time-inhibition curves obtained after dilution. The results obtained in experiments with IPMC and eserine at 25° C are summarized in Table 1. In these experiments the actual dilution was ten times, because of a small volume of aqueous inhibitor that was present in the whole blood. The observed steady state inhibition by eserine has been corrected for the slow enzymatic decomposition of the inhibitor itself which was expected at the relatively high enzyme concentrations used2. This decomposition tends to exaggerate and extend the dilution effect so that reactivation of the diluted enzyme appeared to continue almost to completion after 18 h. Because of its lower carbamylation rate, no correction was required for the steady state inhibition by IPMC, and this did not change significantly over a period of 15 h. The discrepancy between the observed and calculated effects of dilution illustrated in Table 1 may be explained as a partition phenomenon resulting in a lowering of the concentration of free inhibitor in the aqueous phase as follows.
When estimating human blood ChE inhibition as an index of accidental or occupational exposure to insecticidal carbamates it is therefore important to keep z minimal or to carry out the assay so rapidly that the change towards the new steady state can be neglected. Fortunately, the values of K2 for typical insecticidal carbamates2,3 are such that, if assays can be completed within a few minutes of sample dilution, the changes before and during the measurement are relatively small. A simple radiometric method for estimating blood ChE under field conditions has been described4 which enables an assay to be made within 2 min of dilution and involves diluting a 20 µl. blood sample a nominal eleven times. When this method was applied to samples of undiluted human blood pre-incubated with IPMC or eserine to simulate conditions in vivo, it was found that the new steady state effects of subsequent dilution were invariably less than those indicated by equation (2). The steady state inhibition obtaining at the instant of dilution was estimated by extrapolating the time-inhibition curves obtained after dilution. The results obtained in experiments with IPMC and eserine at 25° C are summarized in Table 1. In these experiments the actual dilution was ten times, because of a small volume of aqueous inhibitor that was present in the whole blood. The observed steady state inhibition by eserine has been corrected for the slow enzymatic decomposition of the inhibitor itself which was expected at the relatively high enzyme concentrations used2. This decomposition tends to exaggerate and extend the dilution effect so that reactivation of the diluted enzyme appeared to continue almost to completion after 18 h. Because of its lower carbamylation rate, no correction was required for the steady state inhibition by IPMC, and this did not change significantly over a period of 15 h. The discrepancy between the observed and calculated effects of dilution illustrated in Table 1 may be explained as a partition phenomenon resulting in a lowering of the concentration of free inhibitor in the aqueous phase as follows.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Winteringham, F. P. W., and Disney, R. W., Biochem. J., 91, 506 (1964).
Winteringham, F. P. W., and Fowler, K. S., Biochem. J., 101, 127 (1966).
Reiner, E., and Simeon-Rudolf, V., Biochem. J., 98, 501 (1966).
Winteringham, F. P. W., and Disney, R. W., Lab. Practice, 13, 739 (1964).
Gillette, J. R., in Drugs and Enzymes (edit. by Brodie, B. B., and Gillette, J. R.), 9 (Pergamon Press, London, 1965).
Disney, R. W., Biochem. Pharmacol., 15, 361 (1966).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
WINTERINGHAM, F. Dilution Effects on the Measurement of Blood Cholinesterase Inhibition by Carbamates. Nature 212, 1368–1369 (1966). https://doi.org/10.1038/2121368a0
Issue Date:
DOI: https://doi.org/10.1038/2121368a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.