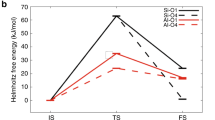
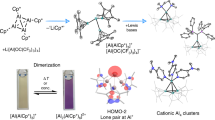
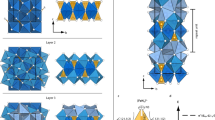
Abstract
ALTHOUGH the crystalline aluminium oxyhalides AlOCl, AlOBr and AlOI are known1–3, the corresponding AlOF is not. We have tried to prepare AlOF by the procedure which is commonly used to prepare the other AlOX compounds, namely, the reaction of 3 AlF3 with As2O3 in sealed evacuated glass ampoules at elevated temperatures. However, AlOF was not formed at temperatures up to 480° C, although reaction between AlF3 and As2O3 did occur. Details of these experiments will be published elsewhere. Whereas AlOCl can be prepared by the reaction of steam and AlCl3 (ref. 4), it does not appear that AlOF can be prepared by a comparable reaction with AlF3. Schober and Thilo5 found that AlF3 reacts with steam at temperatures up to 600° C to form a product with a 5.2 per cent fluorine content, and lower fluorine contents at higher temperatures. They formulated their product as Al7O10F. Locsei6 obtained similar results but preferred to formulate the product as a mixture of Al2O3 and AlOF. No reason was given for the latter choice, but even if it were correct the product would be predominantly Al2O3.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Rouxel, J., Ann. Chim., Paris, 7, Ser. 13, 49 (1962).
Hagenmuller, P., Rouxel, J., David, J., and Colin, A., C.R. Acad. Sci., Paris, 252, 282 (1961); 253, 667 (1961).
Schafer, H., Wittig, F. E., and Wilborn, W., Z. Anorg. Allgem. Chem., 297, 48 (1958).
Lyu, Sub Y., and Zvyagintsev, O. E., Zhur. Prik. Khim., 33, 1208 (1960).
Schober, R., and Thilo, E., Ber., 73B, 1219 (1940).
Locsei, B., Nature, 194, 177 (1962).
Kohlschütter, H. W., Hantlemann, P., Drener, K., and Schilling, H., Z. Anorg. Allgem. Chem., 248, 319 (1941).
Seidell, A., Solubilities (Van Nostrand, New York, 1940).
Daniels, F., Mathews, J. H., and Williams, J. W., Experimental Physical Chemistry, 78 (McGraw-Hill Co., New York, 1941).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
SIEGEL, B., JOHNSON, R. Aluminium Oxyfluoride and the Polymerized Species in AlOCl Solutions. Nature 204, 375–376 (1964). https://doi.org/10.1038/204375b0
Published:
Issue Date:
DOI: https://doi.org/10.1038/204375b0
This article is cited by
-
Oxyfluorides and Basic Fluorides of Aluminium
Nature (1966)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.