Abstract
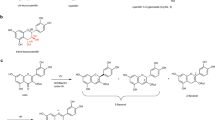
A YELLOW phenolic pigment, which appears on paper chromatograms under ultra-violet light as an apricot-coloured or dull-brown spot changing to a fluorescent yellow-green in ammonia, was first recognized as being present in petals of bearded iris cultivars in 1958. It is probably one of the major co-pigments in Iris flowers, producing, by interaction with the anthocyanin (a delphinidin glycoside), a range of purple, mauve and blue shades1. In an earlier survey of leaf material, the same or a similar substance was observed on chromatograms of all species belonging to the section Pogoniris (which includes the bearded cultivars), of I. pseudacorus Fischer in the Laevigata group of the section Apogon, and of I. dichotoma Pallas in the section Pardanthopsis. This pigment differed in its spectral, chromatographic and colour properties from all the commonly occurring phenolics, such as the flavonoids, and has now been identified as the glycoxan-thone, mangiferin (2-glucosyl-1,3,6,7-tetrahydroxyxanthone) (I), by direct comparison with authentic material. Identification was by means of m.p. and mixed m.p. (268°–270°, decomp.); of the ultra-violet spectra (maxima at 242, 258, 316 and 364 mµ, undergoing characteristic bathochromic shifts in the presence of alkali, aluminium chloride, sodium acetate and sodium acetate–boric acid); of the colour reactions and of co-chromatography in eight different solvent systems. The constituent in the leaves of I. pseudacorus has been examined chromatographically and spectrophotometrically, and appears to be identical with that in the bearded iris. The constituent in I. dichotoma has not been examined in detail. 
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Werckmeister, P., Der Züchter, 24, 224 (1954).
Iseda, S., Bull. Chem. Soc., Japan, 30, 625 (1957).
Hörhammer, L., and Wagner, H., in Recent Developments in the Chemistry of Natural Phenolic Compounds, edit. by Ollis, W. D., 185 (Pergamon Press, 1961).
Hawthorne, B. J., James, N. F., King, F. E., and Morgan, J. W. W., in Chemistry of Natural and Synthetic Colouring Matters, edit. by Gore, T. S., et al., 331 (Academic Press, 1962).
Roberts, J. C., Chem. Rev., 61, 591 (1961).
Bate-Smith, E. C., Sci. Proc. Roy. Dublin Soc., 27, 165 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BATE-SMITH, E., HARBORNE, J. Mangiferin and Other Glycophenolics in Iris Species. Nature 198, 1307–1308 (1963). https://doi.org/10.1038/1981307a0
Issue Date:
DOI: https://doi.org/10.1038/1981307a0
This article is cited by
-
Swertiajaponin fromIris lactea
Chemistry of Natural Compounds (1987)
-
Flavonoids ofHedysarum sericeum andH. caucasicum
Chemistry of Natural Compounds (1983)
-
Mangiferin in some species of the genusHypericum
Chemistry of Natural Compounds (1978)
-
Xanthone glycosides of Iris ensata
Chemistry of Natural Compounds (1974)
-
Über Konstitution und Erbgang eines neuen Delphinidinglycosids „Floridorin“ aus der Garteniris-Sorte cv. ‘Floridor’ (Cayeux 1929)
Der Züchter (1966)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.