Abstract
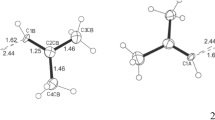
TREATMENT of chloral or its hydrate with concentrated sulphuric acid yields a number of polymeric materials which were identified by Chattaway and Kellett1 and studied by Novak and Whalley2. From the mixture obtained in this Laboratory by treating some 300–400 gm. of chloral hydrate, about 2 gm. (0.5 per cent) of a new polymeric compound was isolated as a white crystalline solid (m.p. 219–220° corr.). It crystallized from absolute ethanol as short needles slightly less soluble in this solvent than chloralide or α-parachloral. The compound is very soluble in acetone, pyridine and ether; moderately soluble in boiling ethanol; slightly soluble in carbon disulphide, carbon tetrachloride and cold ethanol and insoluble in water. Boiling with an aqueous ethanol–20 per cent sodium hydroxide solution liberates chloroform and formic acid. Further work gave the following results: Analysis, calculated for (CCl3–CHO)n: C, 16.28; H, 0.68; Cl, 72.17. Found: C, 16.43; H, 0.73; Cl, 71.78. Molecular weight (Rast), calculated for (CCl3–CHO)4, 589.5. Found: 614, 616, 571 (average 600.3). Infra-red spectrum: the compound was examined in ‘Nujol’ mull (0.025 mm.) and in saturated (18° C.) carbon tetrachloride and carbon disulphide solutions (1 mm.), on a model 137 ‘Infracord’ spectrophotometer (with sodium chloride prism). The bands observed are recorded in Table 1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Chattaway, F. D., and Kellett, E. G., J. Chem. Soc., 2709 (1928).
Novak, A., and Whalley, E., Canad. J. Chem., 36, 1166 (1958).
Barrow, M. G., and Scott, S., J. Amer. Chem. Soc., 75, 1175 (1953).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BARÓN, M. A Cyclic Tetramer of Chloral. Nature 192, 258–259 (1961). https://doi.org/10.1038/192258a0
Issue Date:
DOI: https://doi.org/10.1038/192258a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.