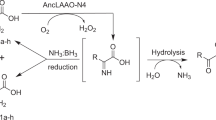
Abstract
IN an earlier communication1, it was reported that a crystal was obtained from the mixture of D-amino-acid oxidase apo-protein, flavin-adenine-dinucleotide and benzoate, a ‘substrate-substitute’. It was also found that the crystal is composed of equimoles of the apo-protein, coenzyme and benzoate. By adding D-alanine to the solution of the crystal, the complete decolorization of the coenzyme accompanied by the liberation of the benzoate was observed. This result indicated that the benzoate can be replaced by the substrate, and that the enzyme is not denatured when crystallized by combining with benzoate. In the present experiment, we sought to find another ‘substrate-substitute’ to crystallize this enzyme.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Yagi, K., Ozawa, T., and Harada, M., Nature, 188, 745 (1960).
Yagi, K., Ozawa, T., and Okada, K., Biochim. Biophys. Acta, 35, 102 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
YAGI, K., OZAWA, T. Crystallization of the Complex of D-Amino-Acid Oxidase and ‘Substrate-Substitute’. Nature 192, 70–71 (1961). https://doi.org/10.1038/192070a0
Issue Date:
DOI: https://doi.org/10.1038/192070a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.