Abstract
THE classical equations of chromatography1 have been applied to the determination of the heat of adsorption of a gas on a solid2,3 by recognizing that the measured residence time, within a packed column, of a pulse of adsorbable gas relative to that of the carrier (or non-adsorbable component) may be related to an ‘equilibrium constant’ K
a by the equation:  where t
a and t
c represent the retention times, respectively, of the active and carrier species. By assuming that K
a ≫ 1 and utilizing the usual temperature functionality for K
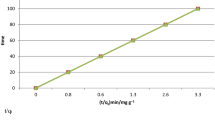
a, Greene and Pust2 show that the logarithm of the retention time of the adsorbing component is linearly related to the reciprocal of the absolute temperature. Thus the observed data on retention time versus temperature permit the evaluation of the adsorption heat, ΔH. Good agreement between values of ΔH determined by this technique and those obtained by others calorimetrically and/or isosterically was found by Greene and Pust for the adsorption of some low boiling gases on charcoal and for light hydrocarbons on alumina and silica gel at temperatures below 100° C. However, these authors correctly noted that no simple correspondence should exist between heats of adsorption derived from continuous-flow transient-response data (adsorption chromatography in their work) and those obtained by calorimetric methods, since the latter are functions of surface coverage.
where t
a and t
c represent the retention times, respectively, of the active and carrier species. By assuming that K
a ≫ 1 and utilizing the usual temperature functionality for K
a, Greene and Pust2 show that the logarithm of the retention time of the adsorbing component is linearly related to the reciprocal of the absolute temperature. Thus the observed data on retention time versus temperature permit the evaluation of the adsorption heat, ΔH. Good agreement between values of ΔH determined by this technique and those obtained by others calorimetrically and/or isosterically was found by Greene and Pust for the adsorption of some low boiling gases on charcoal and for light hydrocarbons on alumina and silica gel at temperatures below 100° C. However, these authors correctly noted that no simple correspondence should exist between heats of adsorption derived from continuous-flow transient-response data (adsorption chromatography in their work) and those obtained by calorimetric methods, since the latter are functions of surface coverage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Martin, A. J. P., and Synge, R. L. M., Biochem. J., 35, 1358 (1941).
Greene, S. A., and Pust, H., J. Phys. Chem., 62, 55 (1958).
Habgood, H. W., and Hanlon, J. F., Canad. J. Chem., 37, 843 (1959)
Carberry, J. J., Amer. Inst. Chem. Eng. J., 4, 13M (1958).
Van Deemter, J. J., Zuiderweg, F. J., and Klinkenberg, A., Chem. Eng. Sci., 5, 271 (1956).
Carberry, J. J., and Bretton, R. H., Amer. Inst. Chem. Eng. J., 4, 367 (1958).
McHenry, K., and Wilhelm, R. H., Amer. Inst. Chem. Eng. J., 3, 83 (1957).
Boudart, M., Amer. Inst. Chem. Eng. J., 2, 62 (1956).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
CARBERRY, J. Determination of Heats of Adsorption by Transient-Response Techniques. Nature 189, 391–393 (1961). https://doi.org/10.1038/189391a0
Issue Date:
DOI: https://doi.org/10.1038/189391a0
This article is cited by
-
Elution curves and statistical moments in non-ideal, linear chromatography
Chromatographia (1984)
-
The median of the chromatographic peak as the best measure of retention time
Chromatographia (1981)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.