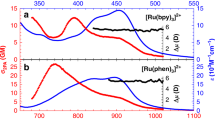
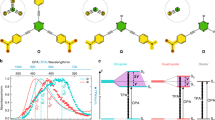
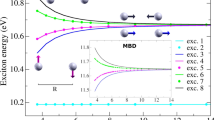
Abstract
IT is generally recognized that non-polar solvents cause a long wave-length shift of the optical spectral maxima of absorbing solutes, and that this shift tends to increase with the polarizability of the solvent. Bayliss1 has proposed that:  where Δν is the long wave-length shift, compared to the dilute vapour spectral position, f is the oscillator strength (that is, the number of ‘classical’ absorbing electrons), a is the radius of the absorbing molecule, n is the refractive index of the solvent, and the other symbols have their usual significance. Longuet-Higgins and Pople2 have proposed the relationship:
where Δν is the long wave-length shift, compared to the dilute vapour spectral position, f is the oscillator strength (that is, the number of ‘classical’ absorbing electrons), a is the radius of the absorbing molecule, n is the refractive index of the solvent, and the other symbols have their usual significance. Longuet-Higgins and Pople2 have proposed the relationship:  where the shift is expressed in energy (frequency × h); αA and αB are the molecular polarizabilities of the solute and solvent, respectively, and M and E are the dipole moment and energy of the transition. Each solute molecule is regarded as being surrounded by z solvent molecules at a mean distance R̄. This relationship predicts that the long wave-length shift will be proportional to (n
2 − l)/(n
2 + 2). Both equations predict that Δνcm.−1) or Δλ (Δλ = λs – λ0, where the subscripts refer to solvent and vapour respectively; over the very short wave-length range to be considered here, Δλ = Δνλ2 to a high degree of approximation) will approach zero only in the vapour state, and that in any solvent a substantial shift should be observed. Similar but more elaborate theoretical treatments have been given by Ooshika3 and McRae4.
where the shift is expressed in energy (frequency × h); αA and αB are the molecular polarizabilities of the solute and solvent, respectively, and M and E are the dipole moment and energy of the transition. Each solute molecule is regarded as being surrounded by z solvent molecules at a mean distance R̄. This relationship predicts that the long wave-length shift will be proportional to (n
2 − l)/(n
2 + 2). Both equations predict that Δνcm.−1) or Δλ (Δλ = λs – λ0, where the subscripts refer to solvent and vapour respectively; over the very short wave-length range to be considered here, Δλ = Δνλ2 to a high degree of approximation) will approach zero only in the vapour state, and that in any solvent a substantial shift should be observed. Similar but more elaborate theoretical treatments have been given by Ooshika3 and McRae4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bayliss, N. S., J. Chem. Phys., 18, 292 (1950).
Longuet-Higgins, H. E., and Pople, J. A., J. Chem. Phys., 27, 192 (1957).
Ooshika, Y., J. Phys. Soc. Japan, 9, 594 (1954).
McRae, E. G., J. Phys. Chem., 61, 562 (1957).
Lauer, K., and Oda, R., Ber., 69, 851 (1936).
Shepard, S. E., Rev. Mod. Phys., 14, 303 (1942).
Phibbs, M. K., J. Chem. Phys., 19, 1430 (1951).
Ham, J., J. Amer. Chem. Soc., 76, 3881 (1954).
Yanari, S. S., and Bovey, F. A., J. Biol. Chem. (in the press).
Matsen, F. A., Ginsberg, N., and Bobertson, W. W., J. Chem. Phys., 13, 309 (1945).
Brice, T. J., in “Fluorine Chemistry”, edit. by Simons, J. H., 436 (Academic Press, New York, 1950).
Simons, J. H., and Dunlap, J. Chem. Phys., 18, 345 (1950).
Sverdlova, O., Optics and Spectroscopy (U.S.S.R.), 6, 223 (1959).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BOVEY, F., YANARI, S. Effect of Solvent Polarizability on the Ultra-Violet Spectral Shifts of Aromatic Compounds. Nature 186, 1042–1044 (1960). https://doi.org/10.1038/1861042a0
Issue Date:
DOI: https://doi.org/10.1038/1861042a0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.