Abstract
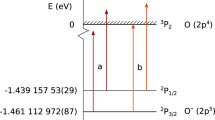
IN a recent communication, Morris and Schmeising1 applied the empirical extrapolation of ionization potentials of isoelectronic ions to determine the binding energy of the O− ion (the atomic oxygen electron affinity). By extending the order of the extrapolation through eight isoelectronic ions (to S7+), they find a slightly higher value, 1.044 eV., than is found from the more usual quadratic extrapolation. Stating that experimental determinations of the electron affinity have given results in unresolved conflict, Morris and Schmeising refer to three discordant values. A value of about 2.2 eV. was obtained by a thermodynamic evaluation of the probability of attachment of electrons to atomic oxygen at a hot filament, and from electron impact studies undertaken originally to determine the dissociation energies of molecular nitrogen and carbon monoxide. The value of 1.48 eV. is given by the optical threshold for photodetachment from O− by Smith and Branscomb. The value of 1.05 eV., which coincides with Morris and Schmeising's extrapolation, was obtained by Schüler and Bingel from the analysis of a certain discrete band spectrum thought to be due to a transition between two bound states of OH−. (For the original references see Morris and Schmeising1.) My purpose here is to resolve these apparent ambiguities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Morris, D. F. C., and Schmeising, H. W., Nature, 181, 469 (1958).
Hagstrum, H. D., J. Chem. Phys., 23, 1178 (1955).
Clarke, E. M., Ph.D. thesis, Univ. Laval, Quebec (1954), communicated to the author by L. Kerwin.
Lagergren, C. R., Ph.D. thesis, Univ. Minnesota (1955) (see note by Branseomb, L., J. Chem. Phys. (in the press)).
Barrow, R. F., and Downie, A. R., Proc. Phys. Soc., A, 69, 178 (1956). Barrow, R. F., Arkiv Fys., 11, 281 (1956).
Schüler, H., and Michel, A., Z. Naturforsch., 11a, 403 (1956). Michel, A., Z. Naturforsch., 12a, 887 (1957).
Branscomb, L. M., Smith, S. J., Burch, D. S., and Geltman, S., Phys. Rev. (in the press).
Branscomb, L. M., “Negative Ions” in “Advances in Electronics and Electron Physics”, 9 (Academic Press, New York, 1957).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BRANSCOMB, L. The Electron Affinity of Atomic Oxygen. Nature 182, 248–249 (1958). https://doi.org/10.1038/182248a0
Issue Date:
DOI: https://doi.org/10.1038/182248a0
This article is cited by
-
Multi-conformational compounds with two absorbing groups. I
Theoretica Chimica Acta (1967)
-
The chemical bond in crystals with an antifluorite structure and the valence possibilities of negative ions
Journal of Structural Chemistry (1967)
-
Electronic structure of compounds AIIBVI with NaCl structure
Journal of Structural Chemistry (1966)
-
Electron Affinity of Oxygen
Nature (1959)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.