Abstract
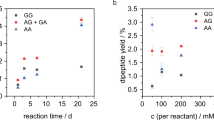
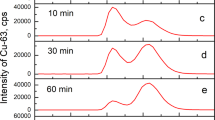
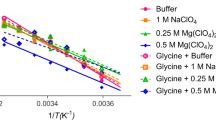
IN the course of an investigation of the amino-acid metabolism of Staphylococcus aureus1, using paper partition chromatography, I used the bromine water oxidation method, devised by Consden and Gordon2, to avoid trouble during the separation of mixtures containing cysteine. The following procedure was used: to a neutralized solution of cysteine and glutamic acid (10 mgm. per ml. of each acid) enough bromine water is added to oxidize all the cysteine to cysteic acid (about 0.1 ml. of bromine water saturated at 20° C. per mgm. of cysteine hydrochloride); the solution is then evaporated to dryness in vacuo at room temperature to remove the slight excess of bromine and the hydrobromic acid formed during the reaction. The residue is dissolved in a small amount of distilled water and dried three times, then made up to the original volume and neutralized. Chromatographic separation on paper (Whatman No. 1 or No. 3), with butanol/acetic acid/water as solvent3, shows, as illustrated on the diagram, four unexpected spots giving slowly a reddish-purple colour with ninhydrin. The two top ones (α1 very weak, and α2, stronger) give, after elution and acid hydrolysis (in 6N hydrochloric acid, 3 hr. at 105° C), strong cysteic acid spots on a new ehromatogram. The two others (β, strong, and γ, weak) run between the two initial amino-acids and are hydrolysed into cysteic and glutamic acids; they move also as single spots in a propanol/water mixture4 (80 : 20, v : v). These spots are not due to incomplete oxidation, since excess of bromine water does not prevent their appearance; working at 0° C. does not affect the result.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gale, E. F., and Van Halteren, M. B. (in the press).
Consden, R., and Gordon, A. H., Biochem. J., 46, 8 (1950).
Woiwod, A. J., J. Gen. Microbiol., 3, 312 (1949).
Hanes, C. S., Hird, F. R. J., and Isherwood, F. A., Nature, 166, 288 (1950).
Consden, R., Gordon, A. H., and Martin, A. J. P., Biochem. J., 41, 590 (1947).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
VAN HALTEREN, M. Artefacts in the Chromatography of Mixtures of Amino-acids containing Cysteine. Nature 168, 1090–1091 (1951). https://doi.org/10.1038/1681090b0
Issue Date:
DOI: https://doi.org/10.1038/1681090b0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.