Abstract
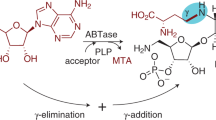
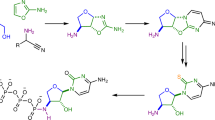
THERE has been isolated from yeast1, crude oryzanine2 and impure cozymase3 an unusual nucleoside which on acid hydrolysis yields adenine and a thiomethyl-sugar. Falconer and Gulland4 claim to have demonstrated that the thiomethyl-sugar and adenine are combined at the 9-position of the base. The thiomethyl-sugar has been the subject of several investigations; thus Suzuki et al.2,5 and Levene and Sobotka6 have shown that the thiomethyl group is retained during osazone formation, thereby excluding positions 1 and 2 of the sugar as the sites where it is combined. The thiomethyl-sugar could be readily reduced to a thiomethylpentitol, which on oxidation with lead tetra-acetate yielded 0.9 mol. of formaldehyde7, indicating that position 3 in the sugar cannot be the combining position of the thiomethyl group. Satoh and Makino8 adduced further evidence which excluded also the C4 of the pentose as the site of the thiomethyl substituent. Previously, Wendt7, using titration data obtained by the Willstätter–Schüdel method, had shown that the thiomethyl-sugar obtained by acidic hydrolysis of the nucleoside was an aldose. Hence all the available evidence indicated that the derivative was a 5-thiomethylpentose.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mandel, J. A., and Dunham, E. K., J. Biol. Chem., 11, 85 (1912).
Suzuki, U., Odake, S., and Mori, T., Biochem. Z., 154, 278 (1924); J. Agric. Chem. Soc. Japan, 1, No. 2 (1924).
Euler, H. von, and Myrbäck, K., Z. physiol. Chem., 177, 237 (1928).
Falconer, R., and Gulland, J. M., J. Chem. Soc., 1912 (1937).
Suzuki, U., and Mori, T., Biochem. Z., 162, 413 (1925).
Levene, P. A., and Sobotka, H., J. Biol. Chem., 65, 551 (1925).
Wendt, G., Z. physiol. Chem., 272, 152 (1942).
Satoh, K., and Makino, K., Nature, 165, 769 (1950).
Raymond, A. L., J. Biol. Chem., 107, 85 (1934).
Weygand, F., Trauth, O., and Löwenfeld, R., Ber., 83, 563 (1950).
Levene, P. A., and Stiller, E. T., J. Biol. Chem., 104, 299 (1934).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
OVEREND, W., PARKER, L. Synthesis of 5-Thiomethylribose. Nature 167, 526–527 (1951). https://doi.org/10.1038/167526b0
Issue Date:
DOI: https://doi.org/10.1038/167526b0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.