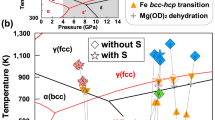
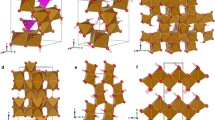
Abstract
PREVIOUS investigations on the solubility of sulphur in iron sulphide (FeS) have led to the conclusion that the solid solutions of sulphur in iron sulphide are formed by substituting some of the iron atoms in the original lattice by sulphur atoms. Assuming that the radius of the sulphur atoms is smaller than that of the iron atoms, this hypothesis explains the fact that the lattice dimensions decrease with increasing sulphur content. It seems, however, doubtful if this relation between the radii of iron and sulphur atoms agrees with reality, and the difficulties are still more increased when one has to explain the analogous (only more pronounced) lattice variations in solid solutions of selenium in iron selenide (FeSe) in the same way, on the assumption that the selenium atoms are smaller than the iron atoms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
HÄGG, G. Vacant Positions in the Iron Lattice of Pyrrhotite. Nature 131, 167–168 (1933). https://doi.org/10.1038/131167b0
Issue Date:
DOI: https://doi.org/10.1038/131167b0
This article is cited by
-
A thermodynamic study of digenite solid solution (cu2-δs) at 600 to 1000°c and a statistical thermodynamic critique on general nonstoichiometry
Metallurgical Transactions B (1976)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.