Abstract
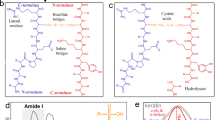
IN a note which appeared recently in these columns, Speakman and Hirst1 recorded comparisons of the work necessary to stretch wool fibres in water, in acids, and after treatment with nitrous acid. The conclusion they reached was that acids disrupt a linkage of the type R1 - COO - NH3 - R2 in the keratin molecule formed from acidic and basic units in adjacent polypeptide chains. Taking the figures quoted by Marston 2 for the amino-acid composition of wool, it was shown that the dibasic aspartic and glutamic acids were collectively present in quantity roughly proportional to that required on the assumption that they entered into a linkage of the type postulated with the e-amino group of lysine and the guanidine group of arginine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Speaksman and Hirst, NATURE, 128, 1073; Dec. 26, 1931.
Marston, Council of Sci. and Ind. Res., Commonwealth of Australia, Bulletin, 38; 1928.
Fischer, "Untersuchungen über Aminosäuren, Polypeptide und Proteine.", 1, 1899–1906; 2, 1907–1919 : Springer, Berlin.
Osborne, J. Amer. Chem. Soc., 24, 140; 1902.
Osborne and Gilbert, Amer. J. Physiol., 15, 333; 1905–1906.
Osborne, Leavenworth and Brautlecht, Amer. J. Physiol., 23, 180; 1908–1909.
Dakin, Biochem. J., 12, 290; 1918.
Luck, Biochem. J., 18, 679; 1924.
Andersen and Röed-Muller, Biochem. Z., 70, 442; 1915.
Vickery and Block, J. Biol. Chem., 86, 107; 1930.
Stewart and Rimington, forthcoming publication in Biochem. J.
Van Slyke and Birchard, J. Biol. Chem., 16, 539; 1913–1914.
Abderhalden and Voitinovici, Z. physiol. Chem., 52, 368; 1907.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RIMINGTON, C. Constitution of the Keratin Molecule. Nature 129, 580–581 (1932). https://doi.org/10.1038/129580c0
Issue Date:
DOI: https://doi.org/10.1038/129580c0
This article is cited by
-
Constitution of the Keratin Molecule
Nature (1932)
-
Constitution of the Keratin Molecule
Nature (1932)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.