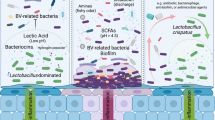
Abstract
The use of chlorhexidine gluconate (CHG) as surgical preparation solution has been advocated due to reduced bacterial loads compared with povidone-iodine (PI). We aimed to investigate changes to the vaginal microenvironment among patients who had laparoscopic hysterectomy and were surgically prepped using 4% CHG compared to 7.5% PI. Premenopausal women who underwent laparoscopic hysterectomy for benign conditions and were prepped with either CHG or PI per surgeon’s choice were enrolled. Vaginal swabs and cervicovaginal lavages were collected prior to vaginal preparation and at 4–6 week post-operative visits for microbiome (α and β diversity, bacterial relative abundances, vaginal pH) and immune marker analyses (protein profiles and concentrations). Antimicrobial activity of clinical CHG and PI formulations were tested in vitro using minimal inhibitory and bactericidal concentration assays. Between February 2021 and June 2022, 41 patients were enrolled. Seven patients either withdrew consent or met exclusion criteria for vaginal bleeding. Thirty-four patients had pre-operative samples collected; 13 patients were lost to follow-up. A total of 21 patients with longitudinal samples of pre- and post-operative collection contributed to this study: 13 in the CHG group and 8 in the PI group. Prior to surgery, 75–77% of women in both groups exhibited Lactobacillus dominance. PI did not change overall vaginal microbiome profiles; however, CHG impacted Lactobacillus iners-dominant profiles, shifting to other lactobacilli (50%) or dysbiotic anaerobes (33%). Lactobacillus crispatus-dominant profiles, which are optimal for vaginal health, were not impacted by either antiseptic solution. In vitro assays further confirmed higher susceptibility of L. iners to CHG solution compared to other vaginal lactobacilli species. Pro-inflammatory cytokines or chemokines were not increased in the CHG or PI group. Our study suggests that CHG does not increase the rate of post-operative vaginal dysbiosis, or genital inflammation compared to PI. Species-specific effects of CHG on vaginal lactobacilli and its clinical impact require further investigation.
Similar content being viewed by others
Introduction
Surgical site infections (SSIs) are a clinically important source of post-operative morbidity and, consequently, healthcare costs1. In gynecologic surgeries, the most common infections include superficial incisional cellulitis, deep incisional abscesses, pelvic abscesses, or vaginal cuff cellulitis2. SSIs occur in ~1–3% of patients after a hysterectomy3. Vaginal cleansing, in addition to abdominal preparation, prior to surgery leads to a decreased rate of SSIs4,5. Povidone-iodine (PI) has been used as an antiseptic since the 1950s6 and is the most used vaginal operative preparation solution in the United States7. While the exact mechanism of its antiseptic properties has not been clearly elucidated its proposed that iodine reacts with bacterial amino acids and fatty acids resulting in the destruction of cellular structures and enzymes8. Alternatively, chlorhexidine gluconate (CHG), not approved by US Food and Drug Administration (FDA) for vaginal preparation, is commonly used as such off-label2,9. This solution causes the destruction of bacterial cell membranes, leading to leakage of intracellular components and coagulation of cell contents8. Although PI is the most common solution used in the US, CHG is the preferred choice in many other countries7.
Recently, the use of CHG over PI has been advocated by medical societies due to increased reduction in bacterial colonization2,10 and infection rates11. Yet, complete adoption of CHG is hindered by label warnings against its use in mucous membranes and concerns of side effects such as vaginal and urethral irritation9,12,13,14. While the vagina does not contain mucous glands, it lacks keratin and thus, like the oral mucosa, is more susceptible to irritation than skin7. Notably, the use of CHG in hand sanitizers was recently banned by the FDA at concentrations lower than used in surgical preparation given concerns for severe allergic reactions15. There is conflicting evidence of the side effects of CHG as a vaginal cleansing solution. A recent randomized controlled study demonstrated worse vaginal and urinary symptoms with the use of vaginal CHG both in the immediate postoperative period and 24–48 h postoperatively13. However, another prospective study showed that CHG is not associated with increased irritation compared to PI as vaginal cleansing solution12.
The effect of CHG use on the vaginal microbiome is unknown. Previously, two in vitro studies, including one by our group, showed that CHG can significantly inhibit growth of health-associated vaginal Lactobacillus species16,17. Yet, clinical studies evaluating impact of CHG antiseptic solution on the healthy constituents of vaginal microbiota in vivo are lacking. This study aimed to investigate changes in the vaginal microenvironment after the use of 4% CHG compared to 7.5% PI. Our clinical findings coupled with in-vitro experimentation, contribute to ongoing discussion on safety of CHG use for vaginal preparation during gynecological surgeries.
Results
Study population
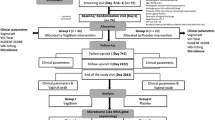
Between February 2021 and June 2022, 41 patients were enrolled. Seven patients either withdrew consent or met exclusion criteria for vaginal bleeding. Thirty-four patients had pre-operative samples collected; 13 patients were lost to follow-up. A total of 21 patients with longitudinal samples of pre- and post-operative collection contributed to this study: 13 in the CHG group and 8 in the PI group. There were no statistically significant demographic differences between the two groups (Table 1). While all our patients received perioperative antibiotic prophylaxis (cefazolin), no patients received antibiotics within the 6 week post-operative timeframe. Additionally, no patients were diagnosed with any post-operative surgical site infection.
Vaginal microbiota diversity following use of chlorhexidine gluconate or povidone-iodine
To examine changes in the vaginal microbiota communities following the vaginal surgical preparation, we performed microbiome analysis using vaginal samples collected prior to the vaginal sterilization (referred as pre-op samples) and 4–6 weeks post-surgery (referred as post-op samples). First, we measured microbiome α-diversity, which summarizes the structure of bacterial communities. Microbial richness (which measures number of taxa within a community) decreased in the PI group (P = 0.04), but not in the CHG group (Fig. 1A). Shannon diversity (which measures both number and distribution of taxa within a community) did not change in either group. Since vaginal pH relates to the vaginal microbiota composition, particularly Lactobacillus dominance (Fig. 1B), we also compared levels of vaginal pH pre- and post-op in each group. No increase in vaginal pH was observed following vaginal preparation with either CHG or PI (Fig. 1C). Then, we measured microbiome β-diversity to assess similarity or dissimilarities of bacterial communities between pre- and post-op samples. We performed a non-metric multi-dimensional scaling (NMDS) and compared the first two coordinates in each group. There was a statistically significant difference in distribution of NMDS2 coordinate between pre- and post-op samples in the CHG group (P < 0.001) (Fig. 2A), indicating changes in overall microbial profiles in this group. However, in the PI group, samples clustered together on the ordination plot and none of the coordinates significantly differed between pre- and post-op samples (Fig. 2B).
A Boxplots show changes in the microbiome richness or Shannon diversity indices post-op compared to pre-op in each group. P-values were calculated using t-test. Boxes extend from the first to the third quartile (Q1 and Q3) with a center line representing the median. Whiskers represent Q1/Q3 ± 1.5 × interquartile range and dots indicate outliers. B Bubble plots show changes in the vaginal pH levels pre- and post-surgery in each group. Bubble size indicates number of patients. pH level ≤ 4.5 is considered normal and associated with Lactobacillus dominance and vaginal health. C Vaginal pH significantly correlated with microbiome richness and diversity.
Non-metric multi-dimensional scaling (NMDS) plots with Bray-Curtis dissimilarity distances show changes in the overall microbial profiles among patients in the CHG (A) and PI group (B). Arrows on NMDS plots indicate shifts in the bacterial profiles for each patient. Boxplots show differences in NMDS1 and NMDS2 for each group. Boxes extend from the first to the third quartile (Q1 and Q3) with a center line representing the median. Whiskers represent Q1/Q3 ± 1.5 × interquartile range and dots indicate outliers.
Vaginal microbiota composition following use of chlorhexidine or povidone-iodine
Relative abundances of bacterial taxa were calculated to analyze the composition of vaginal microbiota in pre- and post-op samples (Fig. 3A). Prior to the vaginal preparation with antiseptic solutions, 16 participants (76%) harbored vaginal microbiota dominated by Lactobacillus species, mostly Lactobacillus iners or Lactobacillus crispatus. At the follow-up visit, seventeen patients (81%) also exhibited Lactobacillus dominance; yet we observed substantial changes in microbiota composition at the species level in patients cleansed with CHG (Fig. 3B). In this group, particularly patients with vaginal microbiota dominated by L. iners transitioned to other Lactobacillus species or bacterial vaginosis-associated bacteria (BVAB). In contrast, we did not observe any major shifts in the vaginal microbiota composition in the PI group. We also performed a differential abundance analysis to identify differences in abundances of individual taxa between pre- and post-op samples in each group. Following the surgical preparation with CHG, we observed significant depletion of L. iners and enrichment of Dialister micraerophilus and Streptococcus anginosus (Fig. 4A). In the PI group, Finegoldia magna was the only species that decreased post-op when compared to pre-op (Fig. 4B).
A Bar plots show taxonomic composition of vaginal microbiota at the species level at pre-op and post-op. Most patients exhibited vaginal bacterial communities dominated by Lactobacillus species prior and after surgery. Asterisks indicate patient with substantial changes in the bacterial profiles. B Alluvial diagrams depict changes of predominant bacteria in microbial profiles pre- and post-op following vaginal preparation with PI or CHG. BVAB: bacterial vaginosis-associated bacteria.
Boxplots show differentially abundant amplicon sequence variants (ASV) between pre-op and post-op in the CHG (A) and PI (B) groups. P-values were calculated using Wilcoxon signed-rank test. Boxes extend from the first to the third quartile (Q1 and Q3) with a center line representing the median. Whiskers represent Q1/Q3 ± 1.5 × interquartile range and dots indicate outliers.
In vitro antimicrobial properties of chlorhexidine and povidone-iodine
Since in vivo data revealed a differential effect of antiseptic solutions on Lactobacillus species, we performed in vitro antimicrobial testing of CHG and PI against the four most common vaginal lactobacilli: L. crispatus, L. iners, L. gasseri and L. jensenii. We used the same antiseptic solutions as routinely used in our hospital (DYNA-HEX 4® Chlorhexidine Gluconate 4% Solution and ScrubCare® 7.5% Povidone-Iodine Scrub) and determined minimal inhibitory concentrations (MIC) of each solution. Both PI and CHG inhibited bacterial growth in a species-specific manner. L. crispatus, L. gasseri, and L. jensenii growth was inhibited at concentrations of 0.00049–0.00098% of CHG (Fig. 5A) and 0.2344–0.4688% of PI (Fig. 5B). However, the transitional species, L. iners, was more sensitive to both antiseptics, with CHG and PI inhibiting its growth at concentrations of 0.00006% and 0.029%, respectively. CHG inhibited Lactobacillus growth at concentrations 30 – 7800 times (1.5–3.9 log) lower than PI and at 4000 – 65500 times (3.6–4.8 log) lower than its original clinically used concentration (4%).
MICs and MBCs of CHG (A) and PI (B) were determine using the broth microdilution method. The ratio of MBC to MIC was used to determine the bactericidal (ratio ≤ 4) or bacteriostatic (ratio > 4) activity of the antiseptics (C). The MIC was defined as the lowest concentration of antiseptic that inhibits all visible growth of the tested Lactobacillus, and the MBC was defined as the lowest concentration of the antiseptic at which 99.9% of the final inoculum is killed on sub-culture. Data shown includes at least 3 independent experiment replicates.
In addition, we determined minimal bactericidal concentration (MBC) of each solution and, by comparing MIC and MBC values, evaluated whether CHG and PI solutions exhibit bactericidal or bacteriostatic activity against tested bacteria (Fig. 5C). PI exhibited bactericidal activity against all tested Lactobacillus species. In contrast, CHG exhibited more bacteriostatic activity against L. crispatus, L. gasseri and L. jensenii. However, both CHG and PI displayed bactericidal activity against L. iners; thus, further demonstrating increased susceptibility of L. iners to these antiseptic solutions compared to other Lactobacillus species.
Immune marker profiles following use of chlorhexidine or povidone-iodine
To identify impact of antiseptic solution on cervicovaginal immune marker profiles, we quantified concentrations of 36 immune mediators, including cytokine, chemokines, and growth factors, in cervicovaginal lavage samples. To visualize relative levels of immune markers and compare global protein profiles between pre- and post-op samples within each group, we created a heatmap. The analysis did not show any substantial increase in levels of immune markers in post-op samples in either group, but it did indicate a decrease in those levels (Fig. 6A). When we compared individual levels of proteins pre- and post-op, we found one chemokine, fractalkine, and one cytokine, interferon α2 (IFNα2), to be decreased in the PI group, and two growth factors, platelet-derived growth factor AA (PDGF-AA) and transforming growth factor α (TGF-α), to be decreased in the CHG group (Fig. 6B). No pro-inflammatory cytokines or chemokines were increased in either group.
A A heatmap reflects relative levels of proteins in cervicovaginal lavages. Data were mean-centered, and variance scaled. The protein profiles were clustered based on the group (CHG and PI) and collection time point (pre-op and post-op). B Scatter plots show concentrations of proteins in individual pre- and post-op samples in the CHG and PI groups. Statistical differences were determined using paired t-test. Only immune markers that were significantly different between pre- and post-op are shown.
Discussion
This pilot study is a translational continuation of previous work that evaluated the impact of clinical and personal lubricants on the local microenvironment of the vagina17,18. While our initial in vitro study revealed distinct alterations in the epithelial cell viability, cytotoxicity, and induction of inflammatory markers with the application of lubricants which related to high osmolality18, in a subsequent study, we demonstrated that lubricants containing CHG as a preservative, as well as pure CHG solution, significantly inhibited growth of vaginal Lactobacillus species in vitro17. This is in accordance with previous clinical studies showing that the use of CHG decreases the vaginal bacterial load at the time of surgery19,20. Overall, these studies demonstrate further need for studying vaginally applied products containing CHG and their effect on the cervicovaginal microenvironment. However, to our knowledge, there are no studies evaluating the delayed impacts of antiseptic solutions, such as CHG, on the vaginal microbiome in vivo. These results suggest that there is no increase in post-operative dysbiosis, characterized as a depletion of health-associated Lactobacillus species, with the use of CHG as a vaginal preparation compared to PI. This is clinically relevant since studies have shown that increased vaginal dysbiosis, also referred to as bacterial vaginosis (BV), is a risk factor for postoperative cuff cellulitis21. Additionally, based on the immune marker profile data, no evidence of genital inflammation was noted following the use of either antiseptic solution as we did not observe elevated levels of proinflammatory cytokines or chemokines in collected samples.
However, we did observe that CHG impacted Lactobacillus iners dominant profiles, which shifted to communities of BV-associated bacteria or other Lactobacillus species. Previous studies have shown that the vaginal microbiome can shift dramatically throughout a women’s lifespan, including puberty, pregnancy, and menopause22. Additionally, the dominant vaginal microbial communities can shift in response to certain triggers, such as menstruation or sexual activity23. In healthy pre-menopausal women, the vaginal microbiome is typically dominated by one or few Lactobacillus species, including L. crispatus, L. iners, L. jensenii or L. gasseri24. Yet, the role of highly prevalent L. iners in vaginal health is still unclear25,26. L. iners can dominate vaginal microbiome of healthy individual but also is detected in women with BV27. Overall, L. iners is considered a transitional community state since it exhibits more instability than other lactobacilli communities28 and consequently can be more vulnerable to disturbances, such as surgical preparation solutions. This increased vulnerability might allow other vaginal microorganisms, such as health-associated L. crispatus or BV-associated bacteria to outcompete L. iners and consequently predominate the microbial community.
This vulnerability of L. iners to antiseptic solutions was observed in the clinical component of our study and confirmed by in vitro experimentation. The use of CHG resulted in the shift of pre-operative L. iners-dominant communities to other Lactobacillus species or dysbiotic bacteria post-operatively. Our in vitro analysis also demonstrated that while the growth of all vaginal Lactobacillus species is impacted by surgical preparation solutions, L. iners is remarkably more susceptible to these solutions, particularly CHG. Our in vitro data confirms that CHG exhibits a species-specific effect on vaginal lactobacilli as we initially observed in our pre- and post-operative clinical sample data. Using the same formulation of CHG used for surgical preparation, CHG exhibited bactericidal activity against L. iners while it demonstrated bacteriostatic activity against the other vaginal Lactobacillus species. Overall, for women with microbiota dominated by L. iners prior to surgery, the disturbance of cervicovaginal microenvironment related to use of CHG for surgical preparation may lead to transition to dysbiosis and, if progressed to clinical BV, increase a risk of post-operative surgical site infections21.
One of the main strengths of this study is the longitudinally matched patient samples that evaluated changes pre- and post-operatively. We also performed in-vitro analysis, which confirmed our clinical data. Additionally, while this study was not randomized, it was prospective in nature which limits confounding factors. One of the major limitations is the small sample size. There was a significant loss of follow-up as well, predominantly due to the COVID-19 pandemic which reduced the post-operative follow up rate. However, the intention of this study was not to provide overwhelming support for the use of one surgical cleansing solution over the other, which would require larger studies. Rather, it was an exploratory study to determine whether chlorhexidine gluconate and povidone iodine alter the vaginal microenvironment post-operatively.
In conclusion, our study suggests that CHG does not increase the rate of vaginal dysbiosis, or genital inflammation compared to PI when used as a vaginal preparation for laparoscopic hysterectomies. Yet, both in our clinical and in vitro datasets, L. iners was more susceptible to application of these vaginal preparations, particularly CHG. Specifically, CHG exhibited bactericidal activity against L. iners, however it had more bacteriostatic activity against the other lactobacilli species. Overall, these findings demonstrate species-specific effects of CHG on vaginal lactobacilli, which warrants further investigation.
Methods
Ethics approval and consent
This single institution, prospective observational study was approved by the University of Arizona Institutional Review Board (IRB No. 2008991024) as well by ClinicalTrials.gov (NCT04658355). The study was conducted at Banner University Medical Center – Phoenix (Phoenix, AZ, USA). All patients provided written informed consent to participate. The study was conducted in accordance with federal guidelines and regulations and the Declaration of Helsinki.
Study population
Participants were identified preoperatively from an ambulatory Minimally Invasive Gynecologic Surgery (MIGS) clinic staffed by three surgeons. Premenopausal women, aged 18 years or older and undergoing laparoscopic (conventional and robotic) hysterectomy for non-malignant indications, were recruited for this study and provided informed consents. Patients were excluded if they were pregnant, had known allergy to CHG and PI, hepatitis or positive HIV carrier status, currently menstruating, or had used any of the following <12 weeks prior to surgery: antibiotics, antifungals, antivirals, topical steroids, or vaginal douches. Women were also excluded if they had a diagnosis of vaginitis or a vulvovaginal disorder within the 6 weeks prior to surgery. These included genital herpes outbreak, bacterial vaginosis, vulvovaginal candidiasis, sexually transmitted infection (gonorrhea, chlamydia, trichomoniasis), vulvar infection, or urinary tract infection.
Sample collection
After preoperative enrollment, patients were taken to the operating room for the planned hysterectomy. Vaginal preparation groups were determined by surgeon’s choice. Two surgeons routinely used chlorhexidine gluconate while one surgeon routinely used povidone iodine. The only exception to this was if the patient had an allergy to the surgeon’s preferred solution and then they utilized the other preparation solution. Vaginal preparation was performed in the standard manner with either DYNA-HEX 4® Chlorhexidine Gluconate 4% Solution (Xttrium Laboratories, Inc., Mount Prospect, IL, USA) or ScrubCare® 7.5% Povidone-Iodine Scrub (CareFusion, San Diego, CA, USA). Both solutions are approved as surgical preparation of the vagina at our institution. Patients who agreed to participate provided clinical specimens (vaginal swab, pH, and cervicovaginal lavage (CVL)) prior to surgical preparation (in the OR) and at 4–6 weeks post-surgery (post-op) during a standard postoperative visit. Patients who had previously met inclusion criteria and enrolled preoperatively but found to have vaginal bleeding at the time of the intraoperative exam were exited from the study. Demographic variables and perioperative data were abstracted from electronic medical records.
Vaginal swabs were collected using a FLOQ Nylon Flocked Swab (COPAN Diagnostics, Murrieta, CA, USA). Vaginal pH was recorded by the clinician using a sterile cotton swab, Hydrion nitrazine paper, and a scale of 4.5–7.0 (Micro Essential Laboratory Inc., Brooklyn, NY, USA). CVLs were collected using 10 ml of sterile 0.9% saline solution (Teknova, Hollister, CA, USA). Following collection, clinical specimens were immediately placed on ice and frozen at −80 °C within 1 h. Vaginal swabs were thawed on ice and processed for DNA isolation. Total DNA was extracted using the DNeasy PowerSoil Pro Kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instruction. CVLs were thawed on ice, clarified by centrifugation (700 g for 10 min at 4 °C) and aliquoted to avoid multiple freeze-thaw cycles. All samples were stored at −80 °C prior to downstream analyses.
16S rRNA gene sequencing and bioinformatic analyses
Microbiome analysis and 16 S ribosomal RNA (rRNA) gene sequencing of DNA samples obtained from vaginal swabs collected prior to the vaginal sterilization (referred as pre-op samples) and 4–6 weeks post-surgery (referred as post-op samples) were performed at the PANDA Core for Genomics and Microbiome Research, University of Arizona (Tucson, AZ, USA). To profile the bacterial community, the hypervariable region 4 (V4) of the 16 S rRNA gene was amplified by PCR using the primers: 515-F (GTGCCAGCMGCCGCGGTAA) and 806-R (GGACTACHVGGGTWTCTAAT) (1). The primers included Illumina adapters, and the reverse primers included an error correcting 12-base pairs (bp) to allow sample demultiplexing. PCR products were purified with the Ultra-Clean PCR Clean-Up Kit (MO BIO Laboratories, Carlsbad, CA, USA), and quantified with the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Waltham, MA, USA). DNA products were pooled together in equimolar concentrations and sequenced on a 2 × 150 bp Illumina MiSeq platform (Illumina, San Diego, CA, USA). Extraction and PCR negative control were included to control for possible contaminants. Demultiplexing was performed using idemp (https://github.com/yhwu/idemp). DADA2 was used for amplicon quality filtering, denoising and chimera removal until amplicon sequence variants (ASVs) were obtained. Taxonomic identities were inferred using the RDP classifier, trained on the Genome Taxonomy Database (GTDB) release 202. Microbiome α-diversity, which summarizes the structure of bacterial communities, was measured using two different indices: observed richness and Shannon diversity. Non-metric multi-dimensional scaling (NMDS) was preformed to measure microbiome β-diversity to assesses similarity or dissimilarities of bacterial communities between pre- and post-op samples.
Quantification of soluble proteins
Levels of 36 proteins (sCD40L, EGF, eotaxin/CCL11, FGF-2, Flt-3L, fractalkine/CX3CL1, G-CSF, GROα/CXCL1, IFNα2, IFNγ, IL-1α, IL-1β, IL-4, IL-6, IL-7, IL-8/CXCL8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IP-10/CXCL10, MCP-1/CCL2, MCP-3/CCL7, MDC/CCL22, MIP-1α/CCL3, MIP-1β/CCL4, PDGF-AA, PDGF-AB/BB, RANTES/CCL5, TGF-α, TNFβ, VEGF-A) were measured in CVL samples using the Milliplex MAP Human Cytokine Chemokine Panel I Magnetic Bead Immunoassay (Millipore, Billerica, MA, USA) in accordance with the manufacturer’s protocols. Data were collected with a Bio-Plex 200 instrument and analyzed using Manager 5.0 software (Bio-Rad, Hercules, CA, USA). A five-parameter logistic regression curve fit was used to determine the concentration. All samples were assayed in duplicate. The concentration values below the detection limit were substituted with 0.5 of the minimum detectable concentration provided in the manufacturer’s instructions.
Bacterial strains and culture conditions
Bacterial strains were obtained from the American Type Culture Collection or the Biodefense and Emerging Infections Research Resources Repository (NIAID, NIH as a part of the Human Microbiome Project) and included four vaginal Lactobacillus strains: L. crispatus JV-V01, L. gasseri JV-V03, L. jensenii JV-V16, and L. iners AB107. L. crispatus, L. gasseri, and L. jensenii were grown on de Man, Rogosa, and Sharpe (MRS) agar or in MRS broth. L. iners was grown on MRS agar or in MRS broth supplemented with 4 mM L-cysteine (Alfa Aesar, Heysham, UK) and 1.1 mM L-glutamine (Acros Organics, Geel, Belgium). All Lactobacillus species were grown at 37 °C under anaerobic conditions, generated with a GasPak EZ Anaerobe Container System. All bacterial culture media were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ, USA).
Minimal inhibitory and bactericidal concentration assays
Minimal inhibitory concentrations (MICs) of CHG and PI antiseptic solutions were assessed using the broth microdilution method. Lactobacillus species were grown overnight on agar plates, then inoculated into the corresponding broth and incubated for ~ 20 h. Two-fold serial dilutions of antiseptics in appropriate broths (50 μL) were aliquoted into respective wells in a sterile 96-well microtiter plate. Bacterial suspensions were adjusted to a final optical density at 600 nm (OD600) of 0.05 and aliquoted (50 μL) into wells with dilutions of antiseptic solutions. Broth without antiseptics or bacterial inoculum were used as growth and sterility controls, respectively. The inoculated microtiter plate was incubated at 37 °C under anaerobic conditions. After a 24 h incubation, the OD600 was recorded using an Infinite 200 Pro M Plex Microplate Reader (Tecan, Männedorf, Switzerland). The MIC was defined as the lowest concentration of antiseptic that inhibits all visible growth of the tested Lactobacillus. Minimum bactericidal concentrations (MBCs) of CHG and PI were determined following the broth microdilution by sub-culturing samples from wells with no visible bacterial growth onto the appropriate agar plates and re-incubating for 48–72 h. The MBC was defined as the lowest concentration of the antiseptic at which 99.9% of the final inoculum is killed on sub-culture. Bactericidal activity was defined as a ratio of MBC to MIC of ≤ 4 and bacteriostatic activity as > 4.
Statistical analyses
Statistical analyses were performed using Prism version 10 (GraphPad, San Diego, CA) unless otherwise stated. Differences in the demographic and socioeconomic variables between the two groups were tested using unpaired t-test for continuous variables and Fisher’s exact test or chi square test for categorical variables. The immune marker data was normalized using the logarithmic transformation. Protein profiles were visualized as a heatmap using ClustVis. Prior to visualization, levels of each protein were mean centered, and variance was scaled. Samples were clustered based on the group and collection timepoint. The statistical differences between the protein concentrations were determined using paired t-test. Statistical analyses of microbiome data were implemented in R (Team R Development Core, 2020). α and β diversity metrics were calculated on rarefied data. Community dissimilarities were tested using permutational multivariate analysis of variance (PERMANOVA) as implemented in the function adonis of the vegan package. Differentially abundant taxa were tested with a paired Wilcoxon rank sum test on differences in relative abundances at the ASV level.
References
Seidelman, J. L., Mantyh, C. R. & Anderson, D. J. Surgical site infection prevention: a review. JAMA 329, 244–252 (2023).
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 195: prevention of infection after gynecologic procedures. Obstet. Gynecol. 131, e172–e189 (2018).
Lake, A. G., McPencow, A. M., Dick-Biascoechea, M. A., Martin, D. K. & Erekson, E. A. Surgical site infection after hysterectomy. Am. J. Obstet. Gynecol. 209, 490.e1–9 (2013).
Schwartz, M. A. et al. Use of vaginal chlorhexidine antisepsis prior to hysterectomy to reduce surgical site infection. Gynecol. Oncol. 154, 193–194 (2019).
Uppal, S. et al. Chlorhexidine-alcohol compared with povidone-Iodine for preoperative topical antisepsis for abdominal hysterectomy. Obstet. Gynecol. 130, 319–327 (2017).
Aronson, J. K. Chapter 24 - antiseptic drugs and disinfectants. in Side Effects of Drugs Annual (ed. Aronson, J. K.) 35, 435–445 (Elsevier, 2014).
American College of Obstetricians and Gynecologists. Committee opinion no. 571: solutions for surgical preparation of the vagina. Obstet. Gynecol. 122, 718–720 (2013).
Wendt, C., Frei, R. & Widmer, A. F. Decontamination, disinfection, and sterilization. Manual Clin. Microbiol. https://doi.org/10.1128/9781555817381.ch13 (2015).
US National Library of Medicine. 4% Chlorhexidine Gluconate Skin Cleansing Kit; US Food And Drug Administration (FDA) Approved Product information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=65f87900-8d23-4fb3-be44-1d116aedac78 (2015).
Stone, R. et al. Enhanced recovery and surgical optimization protocol for minimally invasive gynecologic surgery: an AAGL white paper. J. Minim. Invasive Gynecol. 28, 179–203 (2021).
Darouiche, R. O. et al. Chlorhexidine-alcohol versus povidone-Iodine for surgical-site antisepsis. N. Engl. J. Med. 362, 18–26 (2010).
Al-Niaimi, A. et al. Safety and tolerability of chlorhexidine gluconate (2%) as a vaginal operative preparation in patients undergoing gynecologic surgery. Am. J. Infect. Control 44, 996–998 (2016).
Rastogi, S., Glaser, L., Friedman, J., Carter, I. V. & Milad, M. P. Tolerance of chlorhexidine gluconate vaginal cleansing solution: a randomized controlled trial. J. Gynecol. Surg.36, 13–19 (2020).
Shippey, S. H. & Malan, T. K. Desquamating vaginal mucosa from chlorhexidine gluconate. Obstet. Gynecol. 103, 1048–1050 (2004).
Food and Drug Administration, HHS. Safety and Effectiveness of Consumer Antiseptic Rubs; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. https://www.federalregister.gov/documents/2019/04/12/2019-06791/safety-and-effectiveness-of-consumer-antiseptic-rubs-topical-antimicrobial-drug-products-for (2019).
Rabe, L. K. & Hillier, S. L. Effect of chlorhexidine on genital microflora, neisseria gonorrhoeae, and trichomonas vaginalis in vitro. Sex Transm. Dis. 27, 74–78 (2000).
Łaniewski, P., Owen, K. A., Khnanisho, M., Brotman, R. M. & Herbst-Kralovetz, M. M. Clinical and personal lubricants impact the growth of vaginal lactobacillus species and colonization of vaginal epithelial cells: an in vitro study. Sex Transm. Dis. 48, 63–70 (2021).
Wilkinson, E. M., Łaniewski, P., Herbst-Kralovetz, M. M. & Brotman, R. M. Personal and clinical vaginal lubricants: impact on local vaginal microenvironment and implications for epithelial cell host response and barrier function. J. Infect. Dis. 220, 2009–2018 (2019).
Culligan, P. et al. Bacterial colony counts during vaginal surgery. Infect. Dis. Obstet. Gynecol. 11, 161–165 (2003).
Vorherr, H., Vorherr, U. F., Mehta, P., Ulrich, J. A. & Messer, R. H. Antimicrobial effect of chlorhexidine and povidone-iodine on vaginal bacteria. J. Infect. 8, 195–199 (1984).
Soper, D. E., Bump, R. C. & Hurt, W. G. Bacterial vaginosis and trichomoniasis vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am. J. Obstet. Gynecol. 163, 1016–1021 (1990).
Łaniewski, P. & Herbst-Kralovetz, M. M. Connecting microbiome and menopause for healthy ageing. Nat. Microbiol. 7, 354–358 (2022).
Smith, S. B. & Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 595, 451–463 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. Proc. Natl Acad. Sci. USA 108, 4680–4687 (2011).
Petrova, M. I., Reid, G., Vaneechoutte, M. & Lebeer, S. Lactobacillus iners: friend or foe? Trends Microbiol. 25, 182–191 (2017).
Holm, J. B., Carter, K. A., Ravel, J. & Brotman, R. M. Lactobacillus iners and genital health: molecular clues to an enigmatic vaginal species. Curr. Infect. Dis. Rep. 25, 67–75 (2023).
Carter, K. A., Fischer, M. D., Petrova, M. I. & Balkus, J. E. Epidemiologic evidence on the role of lactobacillus iners in sexually transmitted infections and bacterial vaginosis: a series of systematic reviews and meta-analyses. Sex Transm. Dis. 50, 224–235 (2023).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4, 132ra52 (2012).
Acknowledgements
The authors would like to thank all patients who enrolled in the study. We would like to acknowledge Erika Flores and Elisa Martinez (Department of Obstetrics and Gynecology, University of Arizona College of Medicine-Phoenix) for providing clinical regulatory support and Drs. Gabriele Schiro and Daniel Laubitz (PANDA Core for Genomics and Microbiome Research at Steele Children’s Research Center at University of Arizona) for providing microbiome analysis service. Dr Łaniewski was supported by the Guiding U54 Investigator Development to Sustainability (GUIDeS) program under the award for the Partnership of Native American Cancer Prevention funded by the National Cancer Institute of the National Institutes of Health (U54CA143924).
Author information
Authors and Affiliations
Contributions
P.L., G.S., N.M., and M.HK. conceived and designed the study. Data collection was performed by P.L., G.S., P.C., N.M., and M.HK while G.S. and N.M. were also responsible for patient recruitment. Statistical analysis was performed by P.L., P.C., L.F., and M.HK. P.L., G.S., P.C., and M.HK wrote the main manuscript text. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Łaniewski, P., Smith, G., Crossley, P. et al. Impact of chlorhexidine and povidone-iodine antiseptic solutions on the cervicovaginal microenvironment during laparoscopic hysterectomies: a pilot study. npj Womens Health 2, 16 (2024). https://doi.org/10.1038/s44294-024-00022-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44294-024-00022-2