Abstract
The ability to experience pleasurable sexual activity is important for human health. Receptive anal intercourse (RAI) is a common, though frequently stigmatized, pleasurable sexual activity. Little is known about how diseases of the colon, rectum, and anus and their treatments affect RAI. Engaging in RAI with gastrointestinal disease can be difficult due to the unpredictability of symptoms and treatment-related toxic effects. Patients might experience sphincter hypertonicity, gastrointestinal symptom-specific anxiety, altered pelvic blood flow from structural disorders, decreased sensation from cancer-directed therapies or body image issues from stoma creation. These can result in problematic RAI — encompassing anodyspareunia (painful RAI), arousal dysfunction, orgasm dysfunction and decreased sexual desire. Therapeutic strategies for problematic RAI in patients living with gastrointestinal diseases and/or treatment-related dysfunction include pelvic floor muscle strengthening and stretching, psychological interventions, and restorative devices. Providing health-care professionals with a framework to discuss pleasurable RAI and diagnose problematic RAI can help improve patient outcomes. Normalizing RAI, affirming pleasure from RAI and acknowledging that the gastrointestinal system is involved in sexual pleasure, sexual function and sexual health will help transform the scientific paradigm of sexual health to one that is more just and equitable.
Key points
-
Receptive anal intercourse (RAI) is common worldwide.
-
Pleasurable RAI occurs through stimulation of the perianal or anal nerves and prostate or paraurethral glands, inducing vasodilation, erectile tissue engorgement, anopelvic tissue sensitization, and anal sphincter and pelvic muscular contractions.
-
Patients with a stoma and anorectal stump should be counselled on hygiene and dilator use to minimize infections, maintain anorectal patency, and prevent a permanent stoma, promoting RAI restoration.
-
Antidiarrhoeals, anti-flatulence medications, fibre supplements, lower residue diet to control regularity, avoiding spicy foods, timing meals, and defecation prior to RAI can help control symptoms and relieve distress.
-
Survivors of anal, rectal, and colon cancer and patients with gastrointestinal disease should be counselled on problematic RAI due to anal sphincter, neurovasculature, and prostate or paraurethral gland damage resulting in arousal dysfunction, anodyspareunia or orgasm dysfunctions.
-
Management strategies, including anal dilators for anodyspareunia, anal vibrators for arousal disorders, pelvic floor strengthening for anorgasmia and psychological interventions for decreased desire, should be discussed with patients.
Similar content being viewed by others
Introduction
Maintaining the capacity for consensual and pleasurable sexual activity is an essential component of human health and a fundamental human right1,2,3. Despite being a pleasurable sexual activity4, anal intercourse, particularly receptive anal intercourse (RAI) — defined here as stimulation of the anus by a phallus, finger, object (such as dildo), tongue or mouth — is stigmatized and often not acknowledged by health-care professionals5,6. Moreover, RAI is criminalized and even punishable by death in many countries and territories7,8,9.
Nonetheless, RAI is common worldwide10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27. Up to 81% of cisgender gay and bisexual men10, 20–46% of cisgender women10,28 (including cisgender heterosexual29, lesbian30 and bisexual30 women), 80% of transgender women (or transfeminine people)31,32 and 28% of transgender men (or transmasculine people)33 engage in RAI. For intersex people and individuals with differences of sex development34, RAI is a major source of erogenous sensation and pleasure — particularly for those who had genitopelvic surgery at birth resulting in decreased genital sensations35. Additionally, up to 3% of cisgender heterosexual men have engaged in RAI with a phallus36 (likely underestimated due to associated stigma37), up to 27% of cisgender heterosexual women and 13% of cisgender heterosexual men have engaged in RAI through anal stimulation by tongue or mouth (termed analingus or ‘rimming’) in the past month38, and up to 17% of people have engaged in RAI through a dildo attached to a sexual partner39,40 (termed ‘pegging’)41.
In RAI, the anal canal, perianal skin, prostate or paraurethral glands, erectile tissues, pelvic floor muscles, and supplying neurovasculature facilitate pleasure4,42,43. Diseases of the colon, rectum and anus (including disorders of gut–brain interaction (DGBI), structural diseases such as haemorrhoids, infectious diseases, inflammatory bowel disease (IBD) and malignancies) and their treatments (surgical resections, ostomy formation, anal closures, pelvic radiation and systemic therapies, among others) can adversely affect these anatomical structures and sexual function. However, research has largely equated sexual function with penile erections, vaginal intercourse and reproduction44,45,46,47,48,49,50, neglecting the effects of diseases and their treatments on people who engage in RAI4,36,51. Moreover, RAI is interconnected with diseases of the colon, rectum and anus. For example, anal cancer can develop through human papillomavirus (HPV) transmission during RAI52 and aggressive force during RAI can lead to structural gastrointestinal disorders such as anal fissures53.
Research on problematic RAI — encompassing anodyspareunia (painful RAI), arousal dysfunction, orgasm dysfunction and/or decreased sexual desire — due to gastrointestinal diseases and their treatments is lacking. The National Comprehensive Cancer Network acknowledges that RAI is a risk factor for anal cancer54, that anal cancer is more common in cisgender gay and bisexual men than in the general population54 (with anal cancer incidence being approximately 20–80 times higher in gay and bisexual men than in the general population)55, and that sexual dysfunction is among the most distressing toxic effects of cancer treatment54. Unfortunately, National Comprehensive Cancer Network guidelines fail to acknowledge problematic RAI as a distressing outcome or to offer guidance for engaging in RAI after cancer treatments54. Similarly, a survey of 426 members of the Italian Society of Gastroenterology found that 70% of gastroenterologists never or infrequently discuss sexual dysfunction with their patients; by contrast, 70% of patients believe that their gastroenterologist should be able to manage their sexual dysfunction56. Strikingly, the survey completely omits RAI. To address the health-care inequities for people who engage in RAI, it is necessary for health-care professionals to acknowledge pleasurable and problematic RAI4,56,57.
In this Review, we detail the role of anatomy, neurophysiology and microbiota in pleasurable RAI to provide a framework for understanding problematic RAI. We highlight the influence of DGBI, structural diseases, infectious diseases, inflammatory diseases and malignant diseases of the colon, rectum, and anus as well as their associated treatments on RAI. We then discuss management, mitigation and treatment strategies for disease and treatment-related problematic RAI. Overall, this Review seeks to normalize RAI and transform the scientific paradigm of sexual health to one that is more just and equitable.
Pleasurable RAI
The number of people engaging in RAI (Figs. 1 and 2 and Supplementary Box 1) is increasing58,59,60, including a ~5% per decade increase in the UK58 and Australia59,60, potentially corresponding to worldwide cultural and demographic changes such as an increase in the number of people self-identifying as an individual from a sexual and gender minority community61,62,63. Despite this observation, most health-care workers do not routinely discuss, or even acknowledge, RAI23,64,65 due to a lack of RAI-specific health education66 and the cultural stigma5,6,7,8,9,67. The few existing educational programmes address RAI as it relates to sexually transmitted infections (STIs) and harm reduction5,6.
Schematics represent stereotypical anatomy and are provided for general insights into anatomical structures of complex anatomy. a, Representative sagittal image illustrating the genitopelvic anatomy of a cisgender woman experiencing pleasurable receptive anal intercourse (RAI) with a phallus. During RAI, pressure on the cavernous nerves (located between the vagina and rectum)419, paraurethral glands, cervix, posterior bulboclitoris (clitoris and vestibular bulbs)420,421,422, and anus will elicit pleasure and reflexive external anal sphincter contractions96,97,423. Pressure on the cavernous nerves will induce flow into the vestibular bulbs and cause bulboclitoris enlargement424. Engorgement of the vestibular bulbs and crura will additionally induce pressure on the glans, resulting in pleasure and anal sphincter contractions through the bulbocavernous reflex89,97,425. Thrusting in the anus can cause pressure on the vestibular glands and movement of the bulboclitoris, ultimately inducing pleasure, lubrication and pelvic muscle contractions. Movement of the paraurethral glands and bulboclitoris can further stimulate the surrounding nerves to induce anal sphincter and pelvic muscle contraction91. During receptive intercourse, the bulboclitoris will move and stimulate the surrounding nerves to induce pleasure420. Simultaneously, the clitoral bulbs, along with surrounding pelvic structures, become engorged with blood, stabilizing the vagina, anus and paraurethral glands426. As the pelvic and erectile structures fill with blood and the genitopelvic anatomy fixates and sensitizes, movement during RAI will become more pleasurable426. Sustained repetition can result in orgasm427. b, Representative sagittal image illustrating the genitopelvic anatomy of a cisgender man experiencing pleasurable RAI with a tongue and mouth. During RAI, stimulation of the perianal skin and anus will elicit reflex external anal sphincter contraction, and stimulation and movement of the deep portion of the penis (rather than the pendulous part) and prostate will stimulate the pudendal nerve95 and/or cavernous nerves (located between the prostate and rectum419) and elicit reflex external anal sphincter contractions, causing pleasure, erection, ejaculation and orgasm96,97,423. Sustained repetitive activation of these sensory circuits during RAI can continue to build and intensify, which can ultimately lead to orgasm427. c, Representative sagittal image illustrating the genitopelvic anatomy of a transmasculine person experiencing pleasurable RAI with fingers. In transmasculine people who have undergone metoidioplasty428,429 and/or phalloplasty, pleasure from RAI occurs through the stimulation of cavernous nerves and surrounding erectile tissues, paraurethral tissues, perianal skin, and cervix. For those who have undergone metoidioplasty428,429, tactile stimulation of the neophallus might cause a reflex reaction through the dorsal clitoral nerves. For those who have undergone a phalloplasty430,431,432, direct tactile stimulation of the natal clitoris at the base of the neophallus and indirect stimulation through movement of the phallus might cause a reflex reaction through the tissue flap to the dorsal clitoral nerve anastomoses433,434. d, Representative sagittal image illustrating the genitopelvic anatomy of a transfeminine person experiencing pleasurable RAI with an attachable dildo. The reconstructed neurovascular pedicle flap for neoclitoral sensation (which contains the preserved dorsal penile nerves) and a reconstructed neolabia (which might contain the dorsal penile nerves and posterior scrotal nerve innervation) facilitate pleasurable intercourse for patients with zero-depth and full-depth neovaginas435,436. Stimulation of the prostatic and neoclitoral or neolabial neurovasculature will elicit afferent sensory impulses, with reflex efferent impulses causing contraction of the anal sphincter and pelvic floor muscles. Notably, these muscles (the bulbospongiosus and/or the ischiocavernosus) might be partially or completely resected during vaginoplasty435. Further details are available in Supplementary Box 1. Adapted from ref. 4, Springer Nature Limited.
Schematics represent stereotypical genitopelvic neuroanatomy and are provided for general insights into complex structures in cisgender women and transmasculine people (part a) and cisgender men and transfeminine people (part b). Pressure on the anopelvic structures will elicit pleasure, orgasm and external anal sphincter contraction through distinct neural pathways (red: afferent pathway; blue: efferent pathway), resulting in reflex external anal sphincter contraction, which will place pressure on the finger, phallus, object (for example, dildo, vibrator) or tongue of a partner, increasing dyadic arousal, intimacy and pleasure. Further details are available in Supplementary Box 1. Adapted from ref. 4, Springer Nature Limited.
The absence of RAI (and particularly its relation to pleasure) in medical training and sexual education66 results in a lack of information and resources for people engaging in RAI. Additionally, the systemic omission of RAI from medicine and health care might contribute to patient reluctance to initiate discussions and hesitation to seek health-care guidance on RAI as well as in an increase in potential patient harm during RAI66 and the stigma68, including RAI criminalization5,6,7,8,9, everyday colloquialisms (for example, use of the word ‘butthurt’ to critique an overly sensitive person69,70), and negative judgements of the receptive partner (‘bottom’) but not of the insertive partner (‘top’) in anal intercourse (termed ‘bottom-shaming’66,67,71).
To effectively discuss RAI (Fig. 3) and counsel patients on the effects of gastrointestinal diseases and their treatment on RAI, it is essential to destigmatize and normalize RAI and understand it as it relates to pleasure72 — specifically the genitopelvic anatomy, physiology and gut microbiology involved in facilitating pleasurable RAI.
To address medical inequities for individuals engaging in receptive anal intercourse (RAI), health-care providers should proactively engage in open conversations with colleagues, friends and family to learn inclusive language437 and gain awareness of the diverse lived experiences. Then, when interacting with a patient, one can more easily adapt terminology and tone to ensure patient comfort to prevent potential delays in care438. Discussing sexual orientation, gender identity and sex recorded at birth with a patient is a necessary step before asking about preferred sexual behaviours, gender-affirming hormone therapies and genital anatomy439,440,441,442,443. Gathering this information can not only strengthen the physician–patient relationship but also influence accurate diagnoses and treatment recommendations405,444. If a patient does engage in RAI, centring the conversation on pleasure can enhance patient comfort and encourage disclosure of medical concerns5,6,72. Emphasizing pleasure should include a discussion about disease and treatment effects, supporting informed decision-making and improving quality of life445,446 and health outcomes447,448,449,450. Subsequently, a conversation about best practice and safety during RAI should occur and cover anorectal douching, lubricant, alkyl nitrites (termed ‘poppers’) and sexually transmitted infections (STIs). Educating patients about potential interactions between ‘poppers’ and medications (for example, phosphodiesterase 5 (PDE5) inhibitors) is vital to prevent adverse cardiovascular events. Iso-osmolar lubricants (typically silicone based) might be preferred for RAI as hyperosmolar lubricants (typically water based) can induce epithelial damage and increase risk of bleeding and infection374,375,376. However, caution is advised when using silicone lubricant with silicone objects (for example, dildo). STI screening and treatment should be addressed in relation to sexual practices rather than specific populations451 to ensure inclusive and personalized recommendations375. Importantly, STIs affecting the anorectum might be asymptomatic210, and there might be a risk of transmitting protozoal infections (for example, Giardia intestinalis) and enteropathogenic bacterial infections (for example, Escherichia coli) during RAI through oral–anal stimulation and indirect contamination of objects used during intercourse or fingers222. HPV, human papillomavirus; IBD, inflammatory bowel disease; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis.
Functional anatomy and physiology
Although the exact genitopelvic anatomy might differ among cisgender women, cisgender men, intersex people or individuals with differences of sex development, and gender-expansive individuals (that is, those with gender identities or expressions that are outside the gender binary determined by society), the functional anatomy involved in pleasurable RAI are embryologically homologous and functionally equivalent73 (Figs. 1 and 2 and Supplementary Box 1). Stimulation of the nerves in the perianal skin, anus, erectile tissues, and prostate or paraurethral glands4,74, including the pudendal, hypogastric, pelvic splanchnic nerves and their associated branches74,75, helps facilitate the phases of the RAI sexual response cycle — initial excitement, plateau of arousal, orgasm and resolution76,77.
Sensory stimuli — visual, tactile or other forms — initiate excitement during RAI, leading to increased blood flow to the pelvis, which is maintained by a compensatory increase in heart and respiratory rate78. From in vitro studies of human tissues and in vivo studies with animal models79, it is well established that nitrous oxide production triggers pelvic vascular smooth muscle relaxation, enhancing regional blood flow and pelvic tissue sensitivity80,81. The pelvic muscles, erectile tissues and anus continue to become engorged with blood from branches of the internal pudendal artery82,83, enhancing nutrient delivery crucial for neuron communication84. Pressure on the cavernous nerves, located anterolateral to the anorectum (or neovagina), directly from an object (such as finger, phallus, dildo or tongue) or indirectly through the movement of surrounding structures (such as prostate or paraurethral glands, posterior erectile tissues, and vagina or neovagina) can elicit erectile tissue expansion85,86,87.
As the erectile tissues become engorged with blood, the glans (of the natal bulboclitoris (clitoris and vestibular bulbs)88, penis, neoclitoris or metoidioplasty neophallus) becomes more sensitive and, through the bulbocavernosus reflex, glans stimulation leads to external anal sphincter contractions89,90. Simultaneously direct and indirect pressure on the prostate or paraurethral glands will cause pelvic floor muscular contractions — the bulbospongiosus and ischiocavernosus muscles (deep perineal nerve) and external anal sphincter contraction (inferior anal nerve)91,92. Additionally, stimulation of the afferent inferior anal and perineal nerves (branches of the pudendal nerve)86,93 in the sensate anus (below the pectinate line) and adjacent skin can elicit anal sphincter contractions86,94,95 through the anocutaneous reflex nerve pathway96,97.
In contrast to the anus, rich in nerves that transduce pain and pleasure98, the rectum contains mechanoreceptor nerves that detect and translate rectal stretching into neural output98,99,100. Nevertheless, the rectum is involved in pleasurable RAI as low levels of rectal distension can activate rectal contractions and anal relaxations99,100, promote feelings of ‘rectal fullness’74 (pleasurable for many individuals engaging in RAI)74, and reflexively place pressure on a finger, phallus, attached dildo or tongue of a partner, which can increase dyadic arousal, intimacy and pleasure. As genitopelvic nerve electrical impulse signalling continues, blood flow to genitopelvic anatomy increases, organ sensitivity intensifies and RAI arousal escalates, culminating in orgasm80,81,82.
Gut microbiome and RAI
The gastrointestinal–oral and gut–microbiome axes are an important component of gastrointestinal function and gut–brain communication101. By influencing metabolite levels in the neural, endocrine and inflammatory pathways, including dopamine, serotonin, nitric oxide, hydrogen sulfide and γ-aminobutyric acid, experimental data from in vitro studies have suggested that the gut microbiome can contribute to sexual function101,102. The gut microbiome might also help maintain structural integrity103, preserve anatomical function103 and modulate intestinal permeability104, which can influence sexual health. Gut microbial dysbiosis, or an imbalanced microbiome, has been associated with anatomical abnormalities in the anus (anal sphincter hypertonicity, inflammation, anal pain and anal fistulas103,105) and sexual dysfunction106,107, which is likely multifactorial but could be due to metabolite dysregulation102 as well as anatomical dysfunction from pelvic cross-organ sensitization increasing inflammatory-related intestinal permeability104.
The oral microbiome might additionally affect sexual health108 and the relative abundance of nitrate-reducing bacteria in the oral microbiome might influence nitric oxide production109, essential for sexual arousal110. Thus, it is logical that oral microbial dysbiosis might cause sexual arousal dysfunction108. Awareness of how the gastrointestinal microbiome might be implicated in pleasurable and problematic RAI is important to counsel patients, provide context to disease-related and treatment-related dysfunction, and develop novel technologies.
Engaging in RAI might influence the composition of the gut microbiome111 as well as the oral microbiome112,113,114; yet, it is imperative to emphasize that RAI represents only one singular factor among numerous influences that collectively shape the microbiome of an individual115. The microbiota can be influenced by environmental factors such as pH, availability of nutrients and temperature116. Studies have suggested that people who engage in RAI might exhibit a gut microbiome with a higher ratio of Prevotella to Bacteroides117 (a biomarker of diet and lifestyle, with an increased ratio corresponding to a plant-based diet118), possibly influenced by factors such as a higher fibre diet to facilitate RAI preparation119,120, the presence of Prevotella in semen121, post-RAI irritation122, aggressive douching123 and hyperosmolar lubricant use124.
Nonetheless, it is known that many factors likely contribute to the gut microbiota, including diet or antibiotic exposure, as well as to other aspects of health for which inequitable outcomes exist due to the social determinants of health115. Advances in gut–brain microbiome research have suggested that discrimination, social inequity and minority stress likely have direct influences on the gut–brain microbiome axis125,126,127. Thus, one must be cautious and never presume that the gut microbiota is influenced solely by RAI. Still, future research will hopefully provide insight into the role of the microbiome, if any, in facilitating pleasurable RAI, which might help develop interventions for problematic RAI.
Problematic RAI
Problematic RAI is characterized by pain during or after RAI (anodyspareunia), arousal dysfunction, orgasm dysfunction and/or decreased sexual desire4,128,129,130,131. The aetiology of each dysfunction subdomain is multifactorial and can result from issues with the functional anatomy and physiology involved in pleasure4,128,129. Sexual dysfunction can result from multiple factors, including gastrointestinal diseases, iatrogenic interventions (such as medications, surgery or radiation) and biopsychosocial factors4,128,129 (Fig. 4).
Diagnosing problematic receptive anal intercourse (RAI) requires a comprehensive, patient-centred approach. Establishing trust and comfort is critical; encouraging an open discussion in the patient’s own words will substantially help understand the issue. a, Asking specific questions regarding pain (timing during intercourse, location, quality), arousal (associated phallus-related symptoms), orgasm, desire (body image) and taking a comprehensive medical history can help construct a differential diagnosis. A systematic physical examination should begin with inspection, noting any visible abnormalities (such as skin changes in the genitopelvic area or anus) or devices (such as a stoma) to provide further evidence on issues with sexual desire or pain. Assessing the anus and pelvis for tenderness, swelling, or irregularities and performing focused neurological assessments by evaluating sensation and reflexes (anocutaneous96,97, bulbocavernosus89,90) can assist in differentiating the aetiology (for example, neurological, vascular, structural, psychological). The Digital Rectal Examination Scoring System (DRESS; 0 = no pressure, 3 = normal, 5 = tight) can quantify anal sphincter resting and squeeze tones452. Throughout the exam, patient engagement and communication of findings are key for patient understanding and autonomy. Additionally, it is important to discuss trauma, including intimate partner violence, and note any inconsistencies between history and physical exam196. b, Lab tests and imaging can complement clinical assessment and provide further confirmation if a diagnosis is not yet evident453,454,455,456,457. Ultimately, these steps should identify underlying physiological and anatomical factors contributing to problematic RAI to guide targeted treatment strategies. Laboratory values might reveal imbalances or underlying conditions contributing to decreased sexual desire or other aspects of problematic RAI453,454,455,456,457. Anoscopy173 can assist in the diagnosis of external structural abnormalities (for example, fissure, haemorrhoids) and endoanal ultrasonography can assess internal structural abnormalities, specifically the anal sphincters and intersphincteric plane458. MRI provides a detailed analysis of genitopelvic anatomy, uncovering issues such as pelvic floor dysfunction due to inflammation or other structural abnormalities240. Vascular studies employing Doppler ultrasonography can help evaluate blood flow to erectile and pelvic tissues, crucial for diagnosing issues with arousal459. Using fluoroscopy, MRI defecography can evaluate anorectum and pelvic floor dynamics during defection to provide insight into the neurophysiology of the anal sphincters460. Ultimately, empathetic dialogue, a comprehensive assessment and multidisciplinary collaboration among health-care professionals can enable accurate diagnosis and lead to personalized treatment and support for patients with problematic RAI. CNS, central nervous system; DGBI, disorders of gut–brain interaction; NO, nitric oxide; PEG, percutaneous endoscopic gastrostomy; PPIs, proton pump inhibitors; SSRIs, selective serotonin reuptake inhibitors; STIs, sexually transmitted infections.
Anodyspareunia and painful RAI
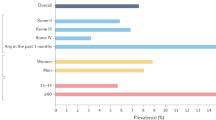
Criteria for the measurement of anodyspareunia — recurrent or persistent pain that occurs before, during or after RAI132 — are varied and include elements similar to those used to measure dyspareunia and vaginismus15,133,134,135. Some have advocated for a standardized clinical definition of anodyspareunia that aligns with genitopelvic pain or penetration disorders15 but criteria used in existing studies vary. A systematic review (identifying 8 studies) notes that the prevalence of anodyspareunia is difficult to assess given varying criteria among studies119; however, using a consistent definition, it is estimated to be from 12% (of n = 277) to 14% (of n = 404) in sexually active cisgender sexual minority men and 9% (of n = 505) in cisgender women engaging in RAI at least twice yearly119, potentially underestimated due to those avoiding RAI due to pain136,137.
Although RAI might initially be painful, over time, the pain generally diminishes and pleasure increases74,138. Anatomical, physiological and psychosocial factors that can contribute to painful RAI include inadequate anorectum lubrication, the anorectal angle, anal sphincter tightness, size of the penetrating object, and lack of relaxation and foreplay133,139,140,141,142. Painful RAI typically occurs at the anus during initial entry (39.6%) or during entry and/or thrusting (16.7%)143. Internal anal sphincter hypertonicity and sphincter spasms can result in difficulty with anal entry and painful RAI65. Sharp pain might additionally be experienced during RAI entry or thrusting if a penetrating object pushes on the rectosigmoid junction causing mesenteric stretching144.
Psychosocial factors, such as generalized or conditioned anxiety, trauma history (more prevalent in women and people from sexual and gender minority communities145), internalized homophobia, fear of STI transmission (including HIV), and fear and/or phobia of engaging in a stigmatized act, can increase sympathetic nervous system activity139,146 and anal sphincter hypertonicity147,148,149. Additionally, defecation concerns, including defecation during RAI, were associated with anodyspareunia in a cohort of cisgender sexual minority men without any particular medical comorbidities engaging in RAI in the 4 weeks prior (n = 135)150. Normalizing and discussing RAI, affirming pleasurable RAI, and offering mitigation strategies can help prevent unnecessary phobias and resultant pain and dysfunction135,143,151.
Arousal dysfunction
Arousal dysfunction can occur from direct damage to the functional erectile and pelvic tissues, injury to the supplying neurovasculature, or impaired blood flow preventing genitopelvic tissue engorgement4,42,152,153. During RAI, people might transiently lose an erection as other areas are stimulated and blood is diverted from erectile tissues; however, diseases and treatment-related sequelae can further shunt blood flow away from pelvic and erectile structures, further decreasing arousal and ultimately delaying orgasm or altogether preventing it4,42.
Orgasm dysfunction
Orgasm dysfunctions include dysorgasmia and anorgasmia4,154. In RAI, the anal sphincters and levator ani are important for facilitating arousal and orgasm155,156 by squeezing an inserted object and helping it apply pressure on the sensate pelvic structures during RAI155. During orgasm, the pelvic floor muscles, including the anal sphincters, contract rhythmically77. Decreased pelvic floor and anal sphincter muscle strength is associated with anorgasmia155,156,157, whereas overactive and inflamed pelvic floor and anal sphincter muscles are associated with dysorgasmia in all people158,159. Thus, damage to the anal sphincters and pelvic floor muscles contributes to orgasm dysfunction in RAI.
Decreased sexual desire
Gastrointestinal diseases and their treatments commonly influence body image and self-esteem, which in turn can negatively affect sexual desire160,161,162. For example, patients with gastrointestinal diseases might have an ostomy or stoma as a result of disease and/or treatment163,164,165,166. The presence of a stoma might influence patient self-perception and inhibit RAI by diminishing the desire for sexual intercourse and intimacy160,161. Reported concerns include anxiety about faecal leakage during intercourse, embarrassment about odour and concern about perception of the stoma by a sexual partner167,168. Among patients with a stoma (n = 540), 51% lived with their stoma for approximately 1–5 years and many described the stoma as negatively influencing their body confidence and sexual desire167. Additionally, in patients with colorectal cancer (CRC; n = 141), compared with patients with no history of an ostomy (n = 98) and patients with a history of a temporary ostomy (n = 18), those with a permanent ostomy (n = 25) have markedly worse body image, even when adjusting for age, sex and depressive symptoms (P < 0.001)168.
Non-malignant gastrointestinal diseases
Non-malignant diseases of the colon, rectum and anus include structural diseases, DGBI, infectious diseases, and inflammatory diseases. It is important for health-care professionals to recognize how each of these disorders and their treatments might affect pleasurable RAI to counsel patients, identify the issue (Fig. 4) and select appropriate management (Table 1).
Structural gastrointestinal diseases
Structural gastrointestinal diseases are common globally169,170, with the exact prevalence and incidence likely being underreported171. Structural gastrointestinal diseases include diseases of the anus (external haemorrhoids, anal fissures, anorectal fistulas), rectum (internal haemorrhoids, rectal prolapse, perirectal abscess) and colon (colonic polyps, diverticular disease)172 (Table 1). Haemorrhoids and anal fissures are among the most common, affecting, for example, at least 13% of the adult population in the USA171.
Anodyspareunia and structural gastrointestinal disorders, such as anal fissures, are indeed strongly linked65. However, in patients who regularly engage in RAI, an anal fissure is likely not from a hypertonic sphincter and likely due to traumatic preparation (enemas, fingers to clean stool, overwiping) or intercourse (inadequate lubricant, aggressive partner)173,174. Before recommending treatment for anal fissures in patients engaging in RAI, a thorough history is necessary as topical calcium channel blockers or botulinum toxin might not be appropriate and conservative management, including increasing fibre, sitz baths and education, might be preferred174.
Before diagnosing idiopathic anodyspareunia, it is important to exclude pain originating from structural gastrointestinal disorders. Utilizing the term ‘genitopelvic pain/penetration disorder’ as a surrogate for anodyspareunia, a cross-sectional ecological study (published as a thesis) found that cisgender sexual minority men who met the study criteria for anodyspareunia (n = 175) were approximately three times more likely to report a history of anal fissures (37%) and 1.5–2 times more likely to report a history of haemorrhoids (48%) than patients who did not meet criteria175. In patients with recurrent anal fissures, chronic ischaemia can result in increased anal sphincter tone and increased anal resting pressure, further predisposing to anodyspareunia176.
Patients with structural gastrointestinal diseases might be predisposed to arousal dysfunction, orgasm dysfunction and/or decreased sexual desire. Haemorrhoids can affect arousal by shunting blood away from erectile tissues177. Pelvic and anal muscular contractions can inhibit venous return from the haemorrhoid plexus, further inhibiting functional blood circulation during RAI177,178. Diverticular diseases might be associated with erectile dysfunction through physical vessel blockage, inflammation and vasculopathies preventing blood flow to erectile tissues152,153. Structural disorders can also influence sexual desire through changes to the physical appearance of the anus, which might result in self-consciousness and decreased desire to engage in RAI179,180.
Disorders of gut–brain interaction
DGBI affecting the colon, rectum and anus include irritable bowel syndrome (IBS), chronic constipation, functional diarrhoea, faecal incontinence, dyssynergic defecation, and functional abdominal bloating and distension, among others172. Similar to structural diseases, prompt recognition of their effects on RAI and appropriate treatment is crucial (Table 1).
Sexual dysfunction in patients with IBS is common, with decreased desire as the most frequently reported manifestation181, occurring in ~21% of participants with IBS (n = 283) in one study182. Decreased sexual desire in IBS is multifactorial, including both physiological and neurotransmitter alterations. Approximately 95% of serotonin is produced in the gastrointestinal tract, which, when dysregulated, can contribute to gut distension, motility and visceral hypersensitivity183,184,185. Patients with IBS have decreased expression of the serotonin reuptake transporter184, which is responsible for regulating serotonin levels184. An increase in serotonin can lead to diarrhoea, discomfort and decreased sexual desire183,184. Patients with IBS are three times more likely to have anxiety or depression than patients without IBS, with an estimated global prevalence of up to 23%186,187. Medications for anxiety and depression, referred to as central neuromodulators in DGBI, can cause adverse effects that include issues with arousal, orgasm and desire188. Before initiating patients on these medications, it is important to assess baseline sexual function to distinguish between subsequent disease effects and medication-related problematic RAI4.
A history of trauma, including sexual abuse, has long been considered a risk factor for patients with IBS and DGBI189,190. Although trauma prevalence varies based on contextual factors, cisgender heterosexual women and people from sexual and gender minority communities are vulnerable to trauma191. Intimate partner violence, which includes physical, sexual and/or emotional abuse perpetuated by a current or former partner or spouse192,193, is another important consideration when diagnosing problematic RAI for a patient with DGBI194. Intimate partner violence is more common among cisgender heterosexual women and people from sexual and gender minority communities192,193. People from sexual and gender minority communities face unique barriers to obtaining support for intimate partner violence, including stigma and structural inequities195. In the context of RAI, intimate partner violence might include a partner refusing to use or secretly removing a condom during intercourse and/or intentionally infecting a victim with an STI (such as HIV) as a means to gain control and power192,193.
A person presenting with problematic RAI with a history of intimate partner violence might complain about DGBI due to anxiety194, anodyspareunia due to a hypertonic anal sphincter139,146,147,148,149, or an anal tear or fissure196. It is important to screen for intimate partner violence in patients presenting for problematic RAI196; screening can include a psychological assessment176, a comprehensive physical exam looking for bruises, bites, cuts, burns or broken bones at various stages of healing (especially in the extremities)196, and an anal exam to look for fissures, tears or bruises and assess sphincter tone139,146,147,148,149. Thus, when discussing and diagnosing problematic RAI, it is important to consider a history of trauma, as trauma is a risk factor for painful RAI197 and pelvic floor muscle dysfunction190,198.
Infections of the colon, rectum and anus
Infectious diseases affecting the colon, rectum and anus that can lead to problematic RAI include condyloma acuminata (from HPV), HIV or AIDS, folliculitis, infectious proctitis, hepatitis, protozoal infections, and enteropathogenic bacterial infections (Table 1).
HPV, which is transmitted through skin–skin contact (including anal–penile and anal–oral RAI), is the most common STI worldwide with an estimated prevalence of 44%199. Condylomata acuminata (anogenital warts) arise in approximately 1% of the global population200,201,202; however, they are twice as common in sexual minority men and can substantially decrease the desire to engage in RAI, whether painful or painless203. Similarly, in cisgender women, anal warts have substantial negative psychosexual effects and can affect desire162. Treatment for condylomata can help alleviate cosmetic concerns; however, local treatments can result in pain during RAI200,201. Condylomata can extend into the intra-anal canal200,201, rich in innervation. As such, treatments for condylomata, including topical and ablative therapies, are frequently associated with pain204,205 and anodyspareunia150. Thus, patients with condylomata should be counselled on the possible risk of treatment-related anodyspareunia.
Patients can acquire and transmit bacterial STIs, including chlamydia, gonorrhoea and syphilis, through RAI206, which can lead to problematic intercourse. If bacterial STIs are untreated, they can cause infectious proctitis207,208, which might be a risk factor for painful RAI, including anodyspareunia209. As STIs of the anorectum (and throat) are more likely to be asymptomatic than those of the genitalia210, it is particularly important to screen patients engaging in RAI for the appropriate STIs. Best practices for STI screening include urinary, oropharyngeal and anorectal swabs (Fig. 3).
It is well established that people living with HIV have an increased risk of sexual dysfunction, and HIV and antiviral therapies can negatively influence RAI by affecting desire and arousal211. Additionally, among cisgender sexual minority men, RAI is the primary route of HIV transmission212. In people living with HIV, issues with desire are likely multifactorial due to diarrhoea (antiretroviral-related opportunistic infections)213, body image changes (antiretroviral-induced lipodystrophy)211 and anxiety (fear of transmission)140,211,214. Anodyspareunia can also affect people living with HIV, reported in up to 18% of cisgender gay and bisexual men living with HIV in one study215. However, HIV itself might not be a risk factor for anodyspareunia as one study found no association between the two150. Yet, a different survey study of sexual minority men found that HIV status was associated with painful intercourse, although no distinction was made regarding the type of pain (anodyspareunia or dysorgasmia)209.
Viral hepatitis can be spread through RAI and could contribute to problematic RAI because damage to the liver and resultant hypoalbuminaemia might decrease sexual desire. The liver regulates albumin production, which is directly correlated to testosterone levels, whereby hypoalbuminaemia from a damaged liver can cause low testosterone, decreased sexual desire216,217 and problematic RAI. Additionally, hepatitis A virus and hepatitis E virus can be transmitted through RAI with a mouth or tongue (oral–anal intercourse) and hepatitis B and D viruses can be transmitted during RAI through bodily fluids218. Hepatitis C virus might be transmitted during RAI if there is active bleeding218.
Additionally, antiretroviral therapy, classically protease inhibitors used for the management of HIV and viral hepatitis, can cause erectile dysfunction211. However, due to inhibiting CYP3A4, protease inhibitors can increase the levels of phosphodiesterase 5 inhibitor concentrations; thus, for patients on protease inhibitors with arousal dysfunction, phosphodiesterase 5 inhibitors should be prescribed at a lower dose and uptitrated219,220,221.
For patients who engage in RAI, protozoal and enteropathogenic bacterial infections can provoke altered bowel habits222, making RAI painful, embarrassing and challenging overall. Engaging in RAI might pose a risk (potentially minor) of transmitting protozoal infections (such as Giardia intestinalis) and enteropathogenic bacterial infections (such as Escherichia coli) through oral–anal contact or through a contaminated object, such as a dildo, as the protozoa and bacteria can be located within microscopic faecal matter222. A case–control study in the USA (case n = 199; control n = 381) identified that, among several risk factors for giardiasis, “male–male sexual behaviour” was among the biggest risk factors for contracting Giardia whereas “female–female sexual behaviour” was not223; however, male–male sexual behaviour does not equate to oral–anal intercourse224. Although oral–anal intercourse might be a potential risk factor for certain infections, it might not be the biggest risk factor.
When evaluating risk factors, it is crucial to be aware of the social determinants of health. For example, in the case of Giardia, access to safe drinking water and sanitation is an important consideration225. Individuals from sexual and gender minority communities encounter unique barriers to accessing safe drinking water and sanitation226, including segregated sanitation facilities based on sex recorded at birth227, histories of trauma or bullying related to bathrooms and locker rooms228, as well as actual or perceived risk of violence in sanitation facilities at shared spaces226. Social determinants of health can substantially contribute to the overall profile for the risk of acquiring an infection, emphasizing the need for a comprehensive understanding beyond assuming that specific sexual behaviours solely contribute to an infection. In addition to advising patients on thoroughly cleansing the anus before anal–oral intercourse, discussing potential use of dental dams during anal–oral intercourse, cleaning sex objects (for example, dildo) after use in an anus, and washing hands after anal stimulation229, it is critical to ask about their ability to access clean water.
Inflammatory bowel disease
Nearly 6.8 million people worldwide live with IBD230, a chronic immune-inflammatory disorder encompassing Crohn’s disease and ulcerative colitis231. Crohn’s disease can affect anywhere along the gastrointestinal tract, including the perianal region in the form of skin tags, fissures, fistulas and strictures232. In ulcerative colitis, inflammation begins in the rectum and extends proximally through the colon in a continuous manner232. Malnutrition and extraintestinal manifestations, including inflammatory disorders of joints, skin and eye, also contribute to the effects of IBD on the quality of life of individuals45,231,232,233,234.
IBD incidence is rising, and it is estimated that it will affect 1% of the global population within the next decade235. IBD symptom onset typically occurs at 15–30 years of age236, with approximately 50% of patients with IBD diagnosed before the age of 35 years45. The prevalence of IBD among older patients (≥60 years old) is increasing rapidly due to a combination of new diagnoses among older adults and an ageing IBD population237. Given the growing incidence and population affected by IBD, understanding the effects of this disease on sexual health is essential45.
Both the disease and its treatments can affect sexual function, including RAI. Sexual dysfunction affects up to 40% of cisgender men and 97% of cisgender women with IBD45. However, there remains a paucity of data to support people with IBD who engage in RAI238. A mixed methods study (n = 50) of cisgender sexual minority men and women with IBD found that these patients experienced fear of judgement related to disclosing their sexuality and sexual practices238. The study highlighted that patients felt ‘robbed’ of their choice to engage in RAI as their gastroenterologists did not discuss RAI nor the effects of IBD and treatments on RAI238. The urgent need to address health inequities in people from sexual and gender minority communities with IBD was additionally identified in a cross-sectional analysis (n = 93) illustrating that individuals from sexual and gender minority communities with IBD have a high risk of mental health comorbidities, social exclusion and unmet nutritional needs239, which can contribute to problematic RAI.
Problematic RAI due to local inflammation
In the anorectal region, IBD can manifest as skin tags, fissures, proctitis, strictures and inflammatory skin disorders; whereas perianal fistulas occur primarily in Crohn’s disease (17–21%240 of patients developing a perianal fistula at 10 years, with incidence increasing with disease duration), they also occur in ~4.5%241 of patients with ulcerative colitis after ileal pouch–anal anastomosis (IPAA)240,242. Perianal disease can be disfiguring and greatly affects quality of life243.
Pain from inflammation and flares might interfere with pleasurable RAI in people with IBD. Proctitis (inflammation of the rectum) is a common manifestation of IBD, occurring in up to 40% of patients with IBD244,245,246, and is associated with rectal pain, tenesmus and bleeding232. For a patient with IBD engaging in RAI, increased bleeding from proctitis might not be life-threating, but could create anxiety for the patient (and partner if present) from concern as well as confusion regarding the underlying aetiology of the bleeding. Additionally, in individuals with prostates affected by IBD with rectal involvement, local inflammation can cause persistent prostatic inflammation217, likely affecting RAI4.
Crohn’s disease-associated perianal fistulas, which are tunnels that occur between the rectum or anal canal and perianal skin, might also contribute to problematic RAI. These lesions are challenging to manage and can be chronic and painful240. The relative position of the fistula (intersphincteric, transphincteric, suprasphincteric or extrasphincteric) might influence sphincter function, treatment type240 and resultant sphincter tonicity247, and therefore RAI.
Perianal fistulas might damage other surrounding structures involved in pleasurable RAI, cause scarring that might make the area feel dry or tight or cause pain during penetration, especially if the fistula is filled with fluid167. Perianal fistulas are also frequently associated with anorectal strictures causing anorectal narrowing248,249, which could limit the ability to engage in RAI, potentially even making penetrative RAI impossible. Additionally, rectovaginal and colo-urethral fistulas can lead to unintentional leakage, causing anxiety surrounding RAI167.
Perianal skin tags are generally benign and can be found in healthy individuals; however, they are more commonly associated with Crohn’s disease250. When enlarged, skin tags can cause idiopathic pain and anatomical blockage251,252,253. Other skin manifestations from inflammation in patients with IBD include erythema nodosum, pyoderma granulosum, anogenital cutaneous Crohn’s disease, and general impaired skin or wound healing243,254, all of which can contribute to pain255,256 and likely anodyspareunia. Although more research is needed, patients with IBD might consider avoiding RAI if pain occurs during penetration and/or if there is active flaring208,257,258,259.
Problematic RAI due to systemic inflammation
Systemic inflammation in patients with IBD can cause fatigue, increased faecal urgency, faecal incontinence, bloody diarrhoea, abdominal pain and malnutrition232,233,236,260, all of which could be associated with problematic RAI. In IBD, fatigue is a common symptom present in up to 50% of patients at diagnosis45. Fatigue can be extremely distressing for patients with IBD and one of the primary contributors to sexual dysfunction261. Patients also report abdominal pain, diarrhoea and the fear of having diarrhoea as contributing to issues with intimacy261.
Problematic RAI due to systemic therapies
Systemic treatments for IBD can influence sexual function45, and likely RAI. Studies investigating the association of steroid use and sexual function in patients with IBD found that prolonged steroid use is associated with decreased sexual satisfaction and pleasure. Prolonged steroid use can lead to adrenal insufficiency, a known risk factor for fatigue and decreased desire262, and body image disturbances, due to acne, fluid retention, obesity, stretch marks and hirsutism45. Patients with IBD identify corticosteroid-related body changes to be most contributory to body image disturbances45,263. Body image dissatisfaction is associated with arousal and orgasm dysfunctions264,265 and, notably, body image might be particularly important for sexual minority men265. Health-care providers should limit the length of steroid use to prevent adverse effects of body image disturbances on sexual health264,265. Biologic therapies to control active inflammation might reduce the need for steroids and the associated adverse effects, helping to prevent steroid-related problematic RAI.
Problematic RAI due to surgical treatments
For people with IBD engaging in RAI who require surgical intervention, a comprehensive discussion on surgical treatment options for IBD and associated risks is essential and should potentially involve their sexual partners (Fig. 5). Rectal inflammation itself can affect RAI but surgical treatments, including bowel resection, fistula repair, diversion procedures and completion proctectomy, can cause complications that further influence RAI such as bleeding, mucus discharge and tenesmus.
a, Effect of inflammatory bowel disease (IBD) resection procedures on receptive anal intercourse (RAI). Restorative proctocolectomy with ileal pouch–anal anastomosis surgery is a gold standard for refractory ulcerative colitis; however, it might affect ability to engage in RAI. The J-pouch, created from ileum and anastomosed to the anus267, might cause problematic RAI if it becomes inflamed (pouchitis) or if it is too small to accommodate an object for RAI. For patients with Crohn’s disease who require colectomy, ileorectal anastomosis208 might be another option, which is associated with fewer bowel movements and night leakages, but increased faecal urgency than ileal pouch–anal anastomosis269. Total proctocolectomy with end ileostomy is reserved for specific cases240, with implications for people who engage in RAI. Patients with IBD might also undergo temporary or permanent ostomy163, which can affect body image, intimacy and intercourse160,161. b, Effect of IBD fistula procedures on RAI. Patients with perianal fistulizing Crohn’s disease often require abscess drainage and seton placement240. Traditional setons can be uncomfortable and affect sexual intimacy167; knotless setons are associated with less pain272, potentially enabling more pleasurable RAI. Fistulotomy can be considered for low intersphincteric fistulas240; however, this approach might create a scar, affecting anorectal elasticity and RAI. Sphincter weakening and stenosis240, which can lead to decreased arousal or anodyspareunia, respectively, are other possible complications from sphincterotomy. In eligible patients, mucosal advancement flap and fistula ligation might be good options to preserve sphincter function and pleasurable RAI.
Intestinal resection and colectomy rates, though slightly declining since the availability of biologic therapies, persist with 7–10% of patients with IBD undergoing one of these procedures266. Restorative proctocolectomy with IPAA, involving the creation of a pouch (called J-pouch) from tissue from the ileum and anastomosis to the anus267, is the gold standard for patients with refractory ulcerative colitis and patients with specific conditions (such as familial adenomatous polyposis).
Qualitative interviews with individuals with IBD from sexual and gender communities reveal how IPAA surgery adversely affected their ability to engage in RAI238. Patients expressed feeling uninformed238 and noted that, while there was guidance on sexual health after surgery, RAI was completely omitted, further affecting their mental health, sexual health and overall quality of life238. Discussing the length of the pouch and pouchitis (inflammation of the J-pouch)267 with people who engage in RAI is likely important — pouch length might affect the ability to accommodate different lengths of objects inserted during RAI259 and pouchitis might make RAI painful.
For patients with Crohn’s disease requiring colectomy, ileorectal anastomosis, which preserves the rectum208, is associated with better sexual function in general, improved fecundity, fewer bowel movements, and fewer night bowel leakages compared with an IPAA268, although it is associated with increased faecal urgency269. It has been reported that hand-sewn anastomosis might be preferred over stapled anastomosis for people engaging in RAI to allow for continued intercourse270. Notably, ileorectal anastomosis is infrequently used in patients with ulcerative colitis due to the potential of persistent rectal inflammation and risk of colitis-associated malignancy268,271.
Patients with IBD might also undergo temporary or permanent ostomy, with up to 10% of patients with Crohn’s disease living with a permanent stoma163, which can affect body image, intimacy and intercourse160,161. Although reserved for select patients, it is important to be aware that total proctocolectomy with end ileostomy, which removes the entire colon and rectum without re-establishment of continuity of the gastrointestinal tract, results in permanent ostomy and would permanently prohibit RAI240, which could substantially and negatively influence quality of life and identity for people who engage in RAI.
In patients with perianal fistulizing Crohn’s disease, fistula management often involves surgical procedures. The most common are abscess drainage and seton placement240. Setons in situ can cause pain and discomfort272 as well as frustration due to decreased sexual spontaneity and difficulties explaining setons to sexual partners167. Knotless setons are associated with significantly less pain (P < 0.001; n = 60)272, possibly enabling more pleasurable RAI. Fistulotomy, typically used for low intersphincteric fistulas that have failed medical management240, carries many risks including scar formation240 (which could reduce anorectal elasticity and the ability to accommodate an object for RAI) and sphincter weakening240 (which could cause decreased arousal). For patients who engage in RAI and are candidates, mucosal advancement flaps and fistula ligation might be preferred options. Mucosal advancement flaps involve mobilizing rectal tissue to cover fistula tracts and preserve the sphincters240; however, the procedure can only be performed in patients without active proctitis and reoperation occurs in up to 50% of patients214. Ligation of intersphincteric fistulas, which has a low risk of faecal incontinence, is another treatment option that might preserve sphincter function240.
Colon, rectal and anal cancer
Maintaining the capacity for sexual pleasure, including pleasurable RAI, after cancer diagnosis and treatment can be fundamental to the quality of life of cancer survivors. Patients with colon, rectal or anal cancer have estimated 5-year overall survivals of 63%, 68% and 70%, respectively273, and advancements in multimodality cancer therapies continue to increase the number of CRC and anal cancer survivors living with treatment-related toxicities44,274,275,276. Among colon, rectal or anal cancer survivors, sexual dysfunction is frequent and distressing, respectively reported at rates of 47% and 28%49, 86% and 72%44, and 40% and 38%50 among male and female survivors.
Colorectal cancer
CRC is the third most common cancer worldwide277. The incidence of CRC can be influenced by oestrogen, an important consideration when managing female, transgender and gender diverse, and intersex patients278,279,280,281,282.
Survivors of CRC might experience iatrogenic sexual dysfunction, including problematic RAI. Approximately 80% of patients with colon cancer undergo surgery283 and patients with rectal cancer are treated with multimodality therapy, including combinations of systemic therapy, radiotherapy and surgery, depending on disease extent and biomarkers284. The probability of a permanent ostomy for patients with CRC who undergo surgery ranges from 10% to 30%164, with up to 21% of patients with rectal cancer who undergo surgery requiring a permanent stoma165. In a survey study of 418 survivors of CRC of varied sexual orientations, 70% of patients reported issues with sexual desire285. When stratified by sexual orientation, a markedly higher proportion of sexual minority patients expressed body image dissatisfaction than heterosexual patients (12% versus 6%; P = 0.04) and a significant association between health-care utilization and sore buttock skin was observed in sexual minority patients but not in heterosexual patients285. Sore buttock skin and body image dissatisfaction can contribute to problematic RAI, highlighting the importance of counselling patients with CRC on these possibilities.
Survivors of CRC experiencing problematic RAI after treatment can encounter inadequate support and feelings of isolation286, which might worsen any treatment-related pain they could be experiencing287. In general, individuals from sexual and gender minority communities experience more loneliness and less familial and social support than the general population288,289,290,291,292. Additionally, they are more prone to experiencing pain during cancer survivorship than their cisgender heterosexual counterparts287. Loneliness triggers similar brain signalling and inflammatory responses as physical pain, and the isolation experienced by individuals from sexual and gender minority communities likely contributes to the observed pain disparity287.
Neglecting conversations about RAI in clinical settings might inadvertently contribute to problematic RAI286 as the omission of RAI-related discussions during cancer care could amplify feelings of isolation, ultimately worsening pain287 and sexual dysfunction. A qualitative analysis (published as a thesis) of semi-structured interviews with six sexual minority men with cancer revealed that the two patients with CRC experienced concerns related to problematic RAI, including pain and relationship breakdowns. However, unlike the three sexual minority men with prostate cancer, the CRC survivors struggled to identify support groups, resulting in more feelings of isolation and loneliness286.
Recognizing sexual health as a crucial component in cancer survivorship, it is essential for clinicians to discuss RAI during CRC care because, by addressing RAI during care and fostering a sense of belonging, clinicians could potentially help mitigate problematic RAI and improve the quality of life for survivors of CRC who engage in RAI.
Anal cancer
Approximately 90% of new anal cancer cases are caused by HPV, often transmitted through sexual intercourse, including RAI293. Despite global efforts in HPV vaccination294,295 and improved anal dysplasia screening296, new anal cancer cases continue to rise worldwide297.
Among the general population, anal cancer is rare; however, among sexual minority men, transgender and gender diverse people, and those with long-term immunosuppression, anal cancer is a relatively common malignancy206,298,299,300,301,302,303,304,305. In the USA, sexual minority men constitute approximately 43% of new anal cancer cases among cisgender men306, with incidence rates ~20–80 times higher in cisgender sexual minority men than in cisgender men and/or women55. Additionally, among gender-expansive individuals, anal cancer ranks as the second most common cancer301.
Sexual minority men with anal cancer are more likely to be single than cisgender heterosexual patients. Mauro et al. found that 60% of sexual minority men with anal cancer were single307, consistent with other studies suggesting that people from sexual and gender minority communities with cancer are more likely to be single than cisgender heterosexual people with cancer287,308, assumed to be proportional to societal tolerance and legal acceptance of same-sex relationships309.
Anal cancer and its treatments can lead to sequelae affecting sexual pleasure4,42,44. In patients with anal cancer, 80% present with locoregional disease310 and are treated with sphincter-sparing concurrent chemotherapy and radiation therapy310. Approximately 10% of patients with anal cancer311 receive an ostomy prior to chemoradiation treatment and, of those patients, at least half go on to live with a permanent ostomy166. Treatment of anal cancer with chemoradiation spares patients the morbidity associated with abdominoperineal resection310; however, it can still adversely affect sexual function312,313,314. For example, in a prospective study evaluating the effects of chemoradiation on quality of life in sexual minority men with anal cancer (mean age: 59.3 years)307, Mauro et al. showed that quality of life and sexual function (specifically penile erectile function) worsened during treatment. Both returned to baseline within a year following treatment completion. However, there were no questionnaires related to the effect of treatment on RAI specifically. To date, no published studies have systematically examined the effect of anal cancer treatments on RAI, and studies are needed to fully elucidate the effect of treatment on RAI.
Surgery and RAI
Surgical resection in patients with CRC can lead to problematic RAI through damage to the neurovasculature and organs responsible for arousal and orgasm4 (Fig. 6). Surgical approaches can also include colostomy, which can affect body image and desire160,161. Nerves implicated in pleasurable RAI can become damaged from surgery, thermal injury, inflammation, ischaemia or stretching315. Patients with colon cancer are treated with a colectomy, which can damage the inferior mesenteric nerves and hypogastric nerves, both of which facilitate sexual, urinary and gastrointestinal function315. Depending on the location of the rectal tumour (low, middle or high), surgical techniques might damage the genitopelvic neurovasculature or even completely remove functional anatomy (including the sphincters)316. For low and middle rectal tumours, sphincter-sparing low anterior resection (LAR) with or without a coloanal anastomosis or abdominoperineal resection with total mesorectal excision might be used, whereas high rectal tumours can undergo a LAR with a higher anastomosis, thus sparing the morbidity associated with extensive resection316.
a, Surgery-related problematic receptive anal intercourse (RAI). Sphincter-sparing surgery is associated with less morbidity than an abdominal perineal resection461. An abdominal perineal resection removes the sphincters and anus, precluding RAI461,462. A low anterior resection with nerve-sparing techniques can be used to reduce sexual dysfunctions, including problematic RAI; however, it can still damage the hypogastric nerves and neurovascular bundle462, adversely affecting arousal463. Ongoing debate exists on removal of the rectoprostatic or rectovaginal fascia for posterior rectal tumours but it is removed during total mesorectal excision for anterior rectal tumours462,464. The rectoprostatic fascia is the location of a neovagina in transfeminine people, and removal of this space would complicate future reconstructive surgery331. Additionally, for anterior tumours, total mesorectal excision has an increased risk of damaging the neurovascular bundle responsible for erectile tissue engorgement during RAI arousal462. Damage to these nerves might also result in low anterior resection syndrome, characterized by increased frequency of bowel movements, tenesmus and faecal incontinence315. The probability of a permanent ostomy for a patient with colorectal cancer undergoing surgery ranges from 10% to 30%164, with potentially 21% of patients with rectal cancer who undergo surgery requiring a permanent stoma165, decreasing sexual desire160,161 and affecting RAI. b, Radiation-related problematic RAI. Radiation contributes to problematic RAI in patients with anal and rectal cancer through long-term anal pain, perianal discomfort, rectal bleeding and faecal incontinence4,42,44,310,313,314. Radiation can damage the functional anatomy involved in pleasurable RAI due to normal functional tissue cell death, inflammation, mucosal ulceration and fibrosis319. Approximately 10% of patients with anal cancer311 receive an ostomy prior to chemoradiation treatment and, of those patients, at least half go on to live with a permanent ostomy, affecting patient self-perception and the desire for sexual intercourse and intimacy166. c, Systemic treatment-related problematic RAI. Systemic therapy, including chemotherapy335,336 and immunotherapy, can influence pleasurable RAI through skin changes, diarrhoea4,150 and resultant malabsorption338, colitis346, and chemotherapy-induced peripheral neurotoxicity340. Immunotherapy is emerging as part of the treatment for colorectal and anal cancers333,344,345. Immunotherapies are associated with diarrhoea and/or colitis307, which might cause anodyspareunia or adrenal insufficiency347, leading to decreased sexual desire and body image dissatisfaction, and may even be associated with vitiligo, which could affect sexual desire465.
Patients with rectal cancer who undergo surgery are likely at increased risk of problematic RAI compared with patients with colon cancer who undergo surgery. A multicentre cross-sectional analysis of survivors of colon cancer (n = 1,145) and rectal cancer (n = 350) showed that LAR syndrome — characterized by increased frequency of bowel movements, tenesmus and faecal incontinence — occurred more frequently in patients with rectal cancer (55%) than in those with colon cancer (21%)317. Despite more survivors of rectal cancer having received neoadjuvant therapy (radiation and/or systemic therapy) than survivors of colon cancer (72% versus 1.7%; P < 0.001), neoadjuvant therapy was not a risk factor for LAR syndrome in this population. Risk factors for this syndrome included female sex and a prior diverting stoma. Additionally, patients who received adjuvant therapy and left hemicolectomy were at lower risk of developing LAR symptoms, further implicating disease location and direct surgical nerve damage as contributing factors317.
The role of non-operative management for rectal cancer is evolving. Patients with rectal cancer managed non-operatively with chemoradiation alone have a lower risk of LAR syndrome than those treated with multimodality therapy. In a cross-sectional survey study of survivors of rectal cancer, patients managed non-operatively with chemoradiation alone (n = 23) experienced fewer LAR syndrome symptoms and less distress than those treated with multimodality therapy (n = 101)318. Using multivariable linear regression, the authors found that surgery was the only statistically significant predictor of worse bowel dysfunction318. Rectal cancer treatments can damage functional anatomy and lead to problematic RAI, especially in patients with low rectal cancers for whom the sequelae can be similar to what is seen after treatment of anal cancer.
Radiation and RAI
Radiation therapy can lead to problematic RAI in patients with anal and rectal cancer through long-term anal pain, perianal discomfort, rectal bleeding and faecal incontinence4,42,44,310,313,314 (Fig. 6). Radiation can damage the functional anatomy involved in pleasurable RAI as a result of normal functional tissue cell death, inflammation, mucosal ulceration and fibrosis319. An observational cohort study of 79 survivors of anal cancer with late treatment toxicity identified that the radiation dose to the hottest 0.5 cm3 of functional anatomy — perianal skin, anal canal and large bowel — was associated with skin toxicity, anal toxicity (for example, sphincter dysfunction) and diarrhoea320.
Patients might experience anodyspareunia due to damage of the sensitive perianal skin and anal canal. Thematic analysis of qualitative interviews with survivors of anal cancer (n = 84) identified problematic RAI as a contributing factor to poor quality of life, for example, one sexual minority male patient discussed his inability to enjoy pleasurable RAI with his husband due to fear of anodyspareunia321. Similarly, prostate-directed radiation has been associated with anodyspareunia through incidental damage to the anus4,15. Given that the anal canal receives a higher dose of radiation during anorectal cancer treatments than prostate cancer treatments, it is important to acknowledge the risk of anodyspareunia when recommending or initiating anal cancer radiation treatments320,322,323.
Radiation can also cause issues with orgasm and arousal by damaging erectile tissue, prostate or paraurethral glands, anal sphincters and anal canal4,42,324. Radiation treatment for anal and rectal cancer includes the prostate as part of its clinical target volumes, which could lead to issues with arousal and orgasm during RAI325. Radiation therapy can damage the anal sphincters and anal canal, especially in patients with large tumours, causing inflammation and fibrosis326,327. Additionally, if the cancer invaded the anal sphincters, sphincter dysfunction can occur even after tumour regression due to initial damage from the tumour. In addition to faecal incontinence, sphincter dysfunction can result in a decreased ability to squeeze a phallus or object for sexual pleasure, further affecting arousal and orgasm in RAI328,329. Fibrosis of the anal canal might cause anal strictures and anal stenosis330, which could lead to painful RAI and anodyspareunia4.
Radiation for anorectal cancer might also influence cosmesis associated with genital-affirming surgery331, influencing the ability and desire of a patient to engage in RAI. For example, a transfeminine person who underwent radiotherapy for anal cancer developed lymphoedema due to the interaction between radiation and injected liquid silicone in the pelvis332. It is important to counsel people experiencing gender incongruence who underwent or are planning to undergo genital-affirming procedures on the implications of radiation treatment on cosmetic outcomes333,334. Moreover, silicone injection is not a standard genital-affirming surgical practice, and it is important for health-care professionals to ask appropriate questions and offer counselling while considering non-standard therapies.
Systemic therapy and RAI
Systemic therapy can negatively affect pleasurable RAI through skin toxicity, malabsorption from diarrhoea and neurotoxicity (Fig. 6). Mitomycin-C335 and 5-fluorouracil336 can cause skin ulceration, skin pain and skin irritation, and 5-fluorouracil can cause diarrhoea337. Diarrhoea can decrease sexual desire4 and cause anodyspareunia150. Malabsorption can lead to pelvic floor muscle weakening and cramps338, causing arousal and orgasm dysfunction155.
Systemic therapy can also affect arousal and orgasm339 through chemotherapy-induced peripheral neurotoxicity340. Chemotherapy-induced peripheral neurotoxicity is among the most common adverse effects, with a prevalence ranging from 19% to 85%, and can markedly affect pleasurable intercourse341. Chemotherapy-induced peripheral neurotoxicity is dependent on agent and dose and particularly common among platinum-based antineoplastics (oxaliplatin and cisplatin), vinca alkaloids (vincristine and vinblastine), epothilones (ixabepilone), taxanes (paclitaxel and docetaxel), proteasome inhibitors (bortezomib) and immunomodulatory drugs (thalidomide)341. Cisplatin, oxaliplatin and other alkylating agents are commonly used for CRC treatment, and secondary nerve damage can result in arousal dysfunction and anorgasmia342. The underlying mechanism for nervous system damage by alkylating agents is neuroinflammation and altered neuron excitability341. Neuroinflammation is caused by the activation of immune cells and an increase in inflammatory cytokines341. These agents also create reactive oxygen species, leading to apoptosis and inflammation. Lastly, chemotherapy can alter the expression of ion channels, including upregulating sodium-gated channels and downregulating potassium-gated channels inducing neuronal hyperexcitability341,343, which might alter sensitivity and therefore influence arousal and orgasm341.
Immunotherapy is emerging as part of the treatment for CRC and anal cancer333,344,345. Immunotherapies are associated with colitis346 (which might cause anodyspareunia) and adrenal insufficiency347 (which is associated with decreased sexual desire and body image dissatisfaction) (Fig. 6). Systemic treatment-related effects can last for many years, and it is important to discuss the lasting effects of systemic therapy on sexual health with patients with CRC or anal cancer.
Treatment choice
Although anal and colon cancers are typically treated with chemoradiation and surgical resection, respectively, rectal cancer is treated with multimodality therapy. As treatment paradigms for cancer continue to evolve, select patients, particularly those with rectal cancer, might be faced with unique treatment choices with variable toxicity profiles348,349,350,351. To guide patients with rectal cancer, physicians should use knowledge of the sexual behaviours of patients, including their preferred role-in-sex (insertive partner (‘top’), receptive partner (‘bottom’), both (‘vers’), neither (‘side’)4) and anatomy. Systematic collection of patient-reported outcomes related to all sexual behaviours, including RAI, will be important to inform future treatment strategies and inform treatment choice decision-making and counselling351.
Management of problematic RAI
Strategies to manage, mitigate and treat problematic RAI can help patients prevent and/or restore the ability to experience pleasure, arousal, orgasm and satisfaction3 (Tables 2 and 3). Although limited evidence exists regarding restorative therapies for pleasurable RAI4 (Tables 2 and 3), it is important to discuss potential therapies for pleasurable RAI rehabilitation. Discussing, characterizing and diagnosing the nature of problematic RAI for a patient (Fig. 3) can help provide insight into optimal strategies to engage in pleasurable RAI safely and confidently.
Engaging in RAI
Generally, if there is no pain, it is safe for patients with non-malignant gastrointestinal disorders to engage in RAI56,172,182 (Table 1). The unpredictability of pain, faecal incontinence, flatulence and/or discomfort208 might cause anxiety and depression regarding RAI. Direct partner communication regarding disease-specific insecurities and symptoms might help alleviate apprehension92,352,353. If it is difficult to receive more rigid objects due to strictures and tightening, pleasurable RAI from the tongue of a partner or soft and small anal beads354 might be good options. However, patients should be counselled on adequate cleaning and preparation to promote partner safety and intimacy.
Practically, antidiarrhoeals, fibre supplements, lower residue diet to control regularity, avoiding spicy foods, timing meals, and defecation immediately prior to intercourse might help control symptoms and relieve mental distress208,355,356. Anti-flatulence medications and/or positioning in the left lateral decubitus position for 15 min in advance of RAI can help expel and prevent unwanted flatulence357,358.
Resumption of RAI
Guidance on resuming RAI after anal, rectal and colon cancer treatments is limited. Counselling patients on safely resuming RAI following treatment is crucial to avoid complications. Extrapolating from the literature and other cancer disease sites, resumption of RAI following treatment should only occur after complete clinical response, complete healing from surgical resection and complete subsidence of radiation-induced inflammation. With regards to anal cancer, a safe approach would likely be approximately 7 months (~30 weeks) after treatment completion, which was identified by adding 4 weeks359 to the 26 weeks that it typically takes for patients with anal cancer to have a complete clinical response310.
Resumption of RAI in survivors of CRC depends on treatment modality. Extrapolating from other disease sites, patients should wait at least 1 month after complete clinical response or treatment completion359, which will likely allow sufficient time for tissue healing and inflammation resolution. Once complete, clinical response is obtained and the anal canal is fully healed (that is, without any anal fissures), survivors of CRC and anal cancer can consider using anal dilators to help remodel scarring and safely stretch the anal and pelvic musculature4,310. Similarly, for patients with non-malignant gastrointestinal diseases who undergo surgery, it is imperative360 to allow the anal canal to heal completely before re-engaging in RAI360. Anal dilators can similarly be introduced to help remodel scar tissue.
Anorectal douching
Anorectal douching should be discussed with cancer survivors and patients with gastrointestinal diseases (Fig. 3). Preparation for RAI with a high-fibre diet and a gentle shower before intercourse should be emphasized; however, patients might still desire douching361. A discussion surrounding douching should focus on minimizing douching-induced damage and not abstinence212.
Health-care professionals should enquire about douching products, timing relative to RAI and delivery method. Plain water (hypo-osmolar) is the most common substance used for anorectal douching361. Additionally, iso-osmolar and hypo-osmolar solutions result in less intestinal damage than hyperosmolar (for example, fleet enemas) solutions as characterized by cell sloughing on histological analysis362. Douching too frequently and/or aggressively, such as with a shower attachment (‘shower shot’)212, can damage the anorectal mucosa and epithelium19,363. A douche enema bulb might be preferable to control the force, especially as self-resolving bleeding can occur in up to 10% of people212. If bleeding occurs, waiting for resolution can decrease infection and further damage. People should limit douching after RAI due to the risk of additional anorectal irritation and infection212, and douching with adequate time before engaging in RAI will allow for residual solution to be expelled361. Counselling patients on the risks of anorectal damage due to product, timing and delivery method is especially important for patients with underlying gastrointestinal disease or long-term treatment sequelae.
STI prevention
Clinicians should discuss appropriate STI screening and prophylaxis with people with gastrointestinal diseases who engage in RAI (Fig. 3). Pre-exposure HIV prophylaxis can be taken as a daily oral pill, such as emtricitabine–tenofovir364, or as long-acting cabotegravir via intramuscular injection administered every 1–2 months365. Gastrointestinal diseases can influence drug pharmacokinetics, including distribution, metabolism and excretion366 as the result of altered gastric emptying time, reduced intestinal length, altered pH, or inflammation impacting permeability366,367. A study investigating the bioavailability of raltegravir in rodents found that pH-altering agents, metal-containing agents and food reduced raltegravir bioavailability368. Similarly, a case study of a patient with a history of HIV and jejunostomy found decreased absorption of orally administered dolutegravir and tenofovir369. Therefore, physicians might consider recommending pre-exposure HIV prophylaxis with long-acting cabotegravir via intramuscular injection for patients with gastrointestinal diseases that influence oral drug absorption365,370.
Furthermore, it is important to discuss doxycycline post-exposure prophylaxis with people who engage in RAI to prevent bacterial STIs (gonorrhoea, chlamydia and syphilis)371. However, although doxycycline post-exposure prophylaxis is safe for cancer survivors and patients with structural and infectious gastrointestinal diseases, patients with IBD and DGBI should be counselled on potential for changes in the microbiome from doxycycline, which could worsen disease372. Furthermore, doxycycline can cause diarrhoea372, which can be distressing for patients who engage in RAI. Still, infectious prophylaxis can likely help to reduce STI transmission-related anxiety and associated issues with sexual desire.
Additionally, discussing condom use during RAI373, potential use of dental dams during anal–oral intercourse229, and sex object and/or finger hygiene during anal intercourse229 is important to prevent STIs. However, acknowledging that what worked for patients before their gastrointestinal disease and associated treatment might no longer be sufficient for pleasurable RAI is recommended as is counselling that patience and tailoring might be necessary due to anatomical and physiological changes. For example, surgical and radiation scarring might increase friction4 and make condom use more difficult. Lubricants can be used to help accommodate an inserted object, including an object covered in a condom4. Due to its ability to adhere to mucosa and scar tissue in addition to minimal drying properties compared with water-based lubricants374,375,376, a silicone-based lubricant might be preferred for RAI4 (Fig. 3).
Engaging in RAI with an ostomy
A stoma might temporarily or permanently prevent patients from engaging in RAI (Figs. 5 and 6). Patients should never insert objects into the stoma, although addressing this aspect with patients should be approached thoughtfully377 (Tables 2 and 3). Patients interested in RAI without a sufficient anorectal stump might experience distress in learning that RAI might not be possible167, and counselling should be offered. Patients with a stoma and an anorectal stump should be counselled on anal dilator use to maintain anorectal patency and help prevent the stoma from becoming permanent378. Additionally, patients should be counselled on cleaning the anorectal stump because it will continue to produce mucus despite the presence of a stoma379.
Patients with a permanent stoma might still have an anorectal stump that is sufficient for RAI. The UK National Health Service cautions against using an anorectal stump for RAI after ostomy formation due to the risk of tearing and bleeding380. However, for many patients, RAI can be extremely important for sexual health and, assuming a sufficient and intact organ, patients might continue engaging in intercourse381. Similar to an anorectal stump, a neovagina can be created from intestine382. For patients with neovaginas, recommendations exist on receptive neovaginal intercourse382, including cleaning with soapy water and maintaining neovaginal patency with dilators383. Given that patients with neovaginas can safely engage in pleasurable receptive neovaginal intercourse, it is likely that patients could safely use an anorectal stump for pleasurable RAI384. For patients without an anorectal stump, research into the creation of a neoanus might be considered to provide patients with the possibility of continuing to engage in pleasurable RAI385. Guidelines and/or consensus statements are necessary regarding the safety and best practices of using an anorectal stump or a neoanus for pleasurable RAI384.
Pelvic floor muscle restoration
The pelvic floor muscles, especially the anal sphincters, are important for arousal, orgasm, satisfaction and pleasure in RAI. Understanding patient symptoms can help guide treatment as a careful balance between pelvic floor stretching, relaxation and strengthening is necessary for pleasurable RAI rehabilitation158,159.
Pelvic floor muscle strengthening
Strengthening the pelvic floor musculature is important for restoring the capacity for orgasm and arousal during RAI. Increased strength of the levator ani muscle, which is connected to the external anal sphincters and penile or clitoral corpus cavernosum and facilitates defecation, bulboclitoris erection, and penile ejaculation, was associated with increased arousal and orgasm157. Researchers hypothesize that increased tone of the vagina facilitates pressure from the inserted object on surrounding sensate structures during intercourse, increasing arousal, orgasm and sexual satisfaction155. Similar to vaginal pressure and tone, anorectal pressure and tone increase during orgasm386 and increased pelvic floor muscle strength helps facilitate pressure from an inserted object on the surrounding sensate structures during RAI, including the bulboclitoris, cavernous nerves, and prostate or paraurethral glands. Strengthening the pelvic floor muscles (including the anal sphincters and levator ani muscles) through Kegel exercises can help restore arousal and orgasm during RAI4 (Table 3).
Sacral nerve stimulator
Sacral nerve stimulation can be used to treat problematic RAI from sphincter dysfunction. Indications include colorectal surgeries (including J-pouch reconstructions), cancer-directed therapies, rectal prolapse and other aetiologies of faecal incontinence387. Implantation of an electrode can re-establish neural pathways and restore sphincter function, strength and control387. For patients with problematic RAI due to functional sphincter control as opposed to anatomic sphincter impairment, sacral nerve stimulation might be a good treatment option388 (Table 3).
Pelvic floor muscle relaxation
There is a complex relationship between biological and psychosocial elements leading to an overactive pelvic floor. Gastrointestinal diseases, surgical interventions and chemoradiation can cause pelvic floor tightness resulting in high tone and/or an overactive pelvic floor, causing pain and impeding arousal by inhibiting blood flow158,159. An overactive pelvic floor has been associated with IBS, illustrating the complex relationship between the pelvic floor, particularly the anal sphincters, and the gut–brain axis158,159.
Pelvic floor tissue flexibility is important for arousal and pain prevention during RAI and orgasm158,159. Physical rehabilitation and behaviour therapy can be used to help with relaxation158,159. A multimodality physical therapy technique incorporating biofeedback, relaxation and stretching was superior to topical lidocaine for the treatment of painful receptive vaginal intercourse in a cohort of cisgender women (n = 212)389. Physical therapy incorporating stretching and biofeedback might help relax pelvic floor muscles and the sphincters to allow for pleasurable RAI (Table 3).
Little evidence exists on medical treatment options for problematic RAI from hypertonic pelvic floor muscles. Diazepam suppositories, botulinum toxin A and muscle relaxants (for example, cyclobenzaprine) have been shown to help with receptive vaginal intercourse158,159. However, more research is needed to confirm the safety of these techniques for the treatment of problematic RAI and caution is needed before administering these treatments (Table 3).
Psychological interventions
For patients experiencing problematic RAI, evaluation by a health psychologist, psychotherapist or certified sex therapist should be considered. Psychotherapy and counselling might start before treatments begin to enable preparation and informed decision-making158,159,390 as many gastrointestinal-directed treatments and, indeed, the gastrointestinal disorders themselves can markedly affect the ability of a patient to engage in pleasurable RAI. A multidisciplinary team coordinating psychological, social behavioural and medical aspects of RAI is ideal for people with gastrointestinal diseases engaging in RAI158,159. Still, a simple empathetic discussion with patients might be effective in decreasing anxiety, alleviating pain and supporting future pleasurable RAI158,159.
Cognitive behavioural therapy
Psychosocial factors can additionally contribute to an overactive pelvic floor and pain158,159. Similar to the relationship between anxiety and pain in receptive vaginal intercourse391, there is a positive correlation between anxiety and pain in RAI134. Anxiety might lead to increased defecation, leakage, flatulence and, subsequently, more anxiety and/or avoidance, creating a cyclical avoidant pattern and further increasing overall hypervigilance, hypertonic pelvic floor muscles and pain186,390,392.
Cognitive behavioural therapy, a highly personalized and collaborative behavioural approach that focuses on remediating maladaptive thoughts, emotions and behaviours, can reduce pain and improve sexual satisfaction. It is one of the most successful gut–brain behaviour therapies for chronic digestive disorders, particularly for those in which the gut–brain interaction is influenced by fear of symptoms393. Cognitive behavioural therapy has been advocated for as a treatment for patients suffering from anodyspareunia394, and might be useful in terminating an anxiety cycle in patients with gastrointestinal diseases to enable pleasurable RAI (Tables 2 and 3).
Gut-directed hypnotherapy
Anatomical changes, such as a J-pouch, from colorectal surgery interventions might make it difficult for patients to connect with their body390,395. Gut-directed hypnotherapy, a therapist-guided relaxation technique with focused suggestions directly related to the symptom or sensation in question (for example, pain) is also a well-tolerated, effective brain–gut behaviour therapy393. Although little is known about its potential to restore healthy brain–gut connections in patients with J-pouches specifically, gut-directed hypnotherapy might be customized to increase positive sensory experiences in the rectum or anus after anatomical changes (Table 2).
Restorative devices
Gastrointestinal diseases, surgical treatments and chemoradiation can cause irritation, pain and friability from inflammation and anal spasms330. It is important to discuss hygienic and safe anal dilation and vibrator practices, including gradually increasing device size396, using a water-based lubricant for sexual device insertion, and cleaning devices with warm water and antibacterial soap to prevent infection397. Ultimately, anal dilators and vibrators might help restore the capacity for pleasurable RAI.
Anal dilators
Anal dilators can help restore anal canal elasticity after treatment for gastrointestinal diseases by stretching and strengthening the anal sphincters (Tables 2 and 3). Anal dilators help minimize anal spasms by stretching the muscles and reducing penetration-associated pain396. Anal dilators can prevent the formation of new scar tissue and help remodel existing scar tissue, shaping the anal canal and promoting anorectal elasticity398,399,400. Anal elasticity, strength and length have important roles in accommodating an inserted object for pleasurable RAI401,402. Anal dilators can also be used to teach relaxation techniques, allowing the patient to gain control over their pelvic floor muscles.
Anal vibrators
Vibrators can help restore reduced sensation from treatment or disease and can be used for arousal and orgasm rehabilitation4 (Table 3). Through stimulation of the prostate or peri-urethral glands, erectile tissues, and surrounding sensitive structures, anal vibrators might be able to restore sensation and pleasure4 following gastrointestinal disease treatments. Even after anatomical alterations or damage, surrounding sensory nerves might be stimulated and enhanced by a vibrator403,404. Thus, vibrators could be useful for patients with gastrointestinal diseases, although more studies are required to confirm their benefit4.
Reframing sexual pleasure
For many patients, RAI can be a substantial component of sexual identity405. Gastrointestinal diseases and treatments can result in a temporary or permanent inability to engage in RAI, thus severely affecting quality of life405. An informed treatment discussion focused on pleasurable affirmation and psychological flexibility is essential. Moreover, a discussion reconceptualizing pleasurable intercourse and sexual activity is important. Framing sexual intercourse around pleasure rather than solely on prevention of negative experiences (or avoidance) is associated with improved health outcomes72,406.
Acknowledging other anatomical erogenous regions in the human body can help reframe sexual pleasure (Tables 2 and 3). Pleasurable regions of the human body, aside from the genitopelvic area, include the mouth or lips, nape of the neck, nipples, and ears81. An anatomical analysis revealed that the non-genitopelvic erogenous zones81, spanning up to 26% of the body surface407, elicit arousal that is even stronger when elicited by a partner rather than self (masturbation)81. Stimulation of these erogenous zones can produce orgasm407. Although the exact neurobiology and neurocircuitry of these areas are poorly understood, evolving research has identified neuroanatomical and psychological bases for sexual pleasure408. Thus, a conversation with patients who are unable to engage in RAI might highlight pleasure from stimulation of other anatomical locations outside the pelvis and from strong interpersonal connections72,407.
Conclusions
RAI is common, and its prevalence will continue to increase with changing global demographics61,62,63 and decreasing stigma. The number of patients with gastrointestinal diseases will also continue to increase235,277,297, furthering the need for effective counselling on the safe and pleasurable practice of RAI. Existing studies illustrate that non-malignant gastrointestinal diseases and treatments for CRC and anal cancer can cause problematic RAI. Disease-related and treatment-related problematic RAI include anodyspareunia, arousal dysfunction, orgasm dysfunction and decreased sexual desire4. The physiology and anatomy involved in RAI are well described; however, more studies are needed to understand the pathophysiology underlying disease-related and treatment-related problematic RAI. Understanding the mechanisms of treatment-related damage will enable researchers to develop novel ways for alleviating problematic RAI, further helping to correct the inequities in scientific and biomedical research43.
Effective communication between clinicians and patients is crucial to mitigate the repercussions of problematic RAI associated with malignant and non-malignant gastrointestinal diseases. Clinicians must ask patients about sexual orientation, gender identity, and sex recorded at birth44,254, and discussions with patients with gastrointestinal diseases should incorporate sexual behaviours, especially RAI, and how disease-related or treatment-related complications might affect sexual pleasure. Centring conversations regarding sexual behaviours on pleasure will help guide conversations, shared decision-making and treatment selection4.
Malignant and non-malignant gastrointestinal disease research and clinical trials must incorporate sexual orientation, gender identity and RAI questionnaires to elucidate disease or treatment effects on RAI409,410,411. As treatment options increase333,348,349,350,351, these questionnaires will be crucial to provide clinicians with evidence for discussing anatomy, physiology and pathophysiology of organ function related to disease-specific and treatment-specific problematic RAI. Additionally, these data could further empower cisgender women, sexual minority men, gender-expansive people and intersex individuals to advocate for more equitable care and research. With these data, RAI can become integrated into medical education and training, further destigmatizing and normalizing sexual behaviours for all people5,6. Gastroenterologists, surgical oncologists, radiation oncologists, medical oncologists and clinical oncologists must expand their definition of sexual activity focused on insertive vaginal intercourse and reproductive ability to include sexual pleasure from all sexual behaviours, including RAI. Health-care professionals must recognize the anorectum as a sexual organ and that the gastrointestinal system is involved in sexual pleasure, sexual function and sexual health to provide basic human rights for all patients, including those who engage in RAI.
References
World Health Organization. Sexual and Reproductive Health and Research (SRH). Including the Human Reproduction Special Programme (HRP). WHO https://www.who.int/teams/sexual-and-reproductive-health-and-research-(srh)/overview (2023).
Ford, J. V. et al. The world association for sexual health’s declaration on sexual pleasure: a technical guide. Int. J. Sex. Health 33, 612–642 (2021).
Dewitte, M. & Reisman, Y. Clinical use and implications of sexual devices and sexually explicit media. Nat. Rev. Urol. 18, 359–377 (2021).
Dickstein, D. R. et al. Sexual health and treatment-related sexual dysfunction in sexual and gender minorities with prostate cancer. Nat. Rev. Urol. 20, 332–355 (2023).
Kutner, B. A., Simoni, J. M., DeWitt, W., Gaisa, M. M. & Sandfort, T. G. M. Gay and bisexual men who report anal sex stigma alongside discomfort discussing anal sex with health workers are less likely to have ever received an anal examination or anal swab. LGBT Health 9, 103–113 (2022).
Kutner, B. A., Wu, Y., Balán, I. C. & Meyers, K. “Talking about it publicly made me feel both curious and embarrassed”: acceptability, feasibility, and appropriateness of a stigma-mitigation training to increase health worker comfort discussing anal sexuality in HIV services. AIDS Behav. 24, 1951–1965 (2020).
Human Rights Watch. World Report 2022: Events of 2021 (Human Rights Watch, 2022).
Bhandari, A. Uganda’s anti-gay bill is the latest and worst to target LGBTQ Africans. Reuters https://www.reuters.com/graphics/UGANDA-LGBT/movakykrjva/ (2023).
Mendos, L. R., Botha, K., Carrano Lelis, R., López de la Peña, E., Savelev, I. & Tan, D. State-sponsored Homophobia. Global Legislation Overview Update. ILGA https://www.ecoi.net/en/file/local/2044751/ILGA_World_State_Sponsored_Homophobia_report_global_legislation_overview_update_December_2020.pdf (2020).
Garros, A. et al. Risk of fecal incontinence following receptive anal intercourse: survey of 21,762 men who have sex with men. J. Sex. Med. 18, 1880–1890 (2021).
Meuwly, M., Auderset, D., Stadelmann, S., Suris, J. C. & Barrense-Dias, Y. Anal intercourse among heterosexual young adults: a population-based survey in Switzerland. J. Sex. Res. 58, 1061–1068 (2021).
Magno, L. et al. Gender-based discrimination and unprotected receptive anal intercourse among transgender women in Brazil: a mixed methods study. PLoS ONE 13, e0194306 (2018).
Kumar, B. & Ross, M. W. Sexual behaviour and HIV infection risks in Indian homosexual men: a cross-cultural comparison. Int. J. STD AIDS 2, 442–444 (1991).
McBride, K. R. Heterosexual women’s anal sex attitudes and motivations: a focus group study. J. Sex. Res. 56, 367–377 (2019).
Wheldon, C. W. et al. Unrecognized sexual dysfunction in gay and bisexual men after prostate cancer treatment: the antecedents and impact of anodyspareunia. J. Sex. Med. 20, 515–524 (2023).
Maierhofer, C. N., Lancaster, K. E. & Turner, A. N. Anal sex is more common than having a twitter account in the United States. Sex. Transm. Dis. 45, 783–785 (2018).
Caballero-Hoyos, R., Monárrez-Espino, J., Ramírez-Ortíz, M. G. & Cárdenas-Medina, F. M. Factors associated with unprotected anal sex among men who have sex with men in Mexico. Infect. Dis. Rep. 14, 547–557 (2022).
Meng, X. et al. Relative risk for HIV infection among men who have sex with men engaging in different roles in anal sex: a systematic review and meta-analysis on global data. AIDS Behav. 19, 882–889 (2015).
Galea, J. T. et al. Rectal douching prevalence and practices among peruvian men who have sex with men and transwomen: implications for rectal microbicides. AIDS Behav. 20, 2555–2564 (2016).
Carlos, S. et al. Heterosexual oral and anal sex in Kinshasa (D.R.Congo): data from OKAPI prospective cohort. PLoS ONE 14, e0210398 (2019).
Morhason-Bello, I. O. et al. Heterosexual oral and anal sex: perceptions, terminologies, and attitudes of younger and older adults in Ibadan, Nigeria. Arch. Sex. Behav. 52, 161–175 (2023).
Mazeingia, Y. T., Olijjira, L. & Dessie, Y. Anal sexual experience and HIV risk awareness among female sex workers in Dire Dawa, eastern Ethiopia. Glob. Health Res. Policy 2, 27 (2017).
Hirst, J., Pickles, J., Kenny, M., Beresford, R. & Froggatt, C. A qualitative exploration of perceptions of anal sex: implications for sex education and sexual health services in England. Cult. Health Sex. 25, 241–255 (2023).
Xia, D. et al. Psychosocial problems and condomless anal sex among transgender women in two cities of China: study based on the syndemic framework. Int. J. Environ. Res. Public Health 19, 16161 (2022).
Parchem, B., Aguayo-Romero, R. A., Alizaga, N. M., Poppen, P. J. & Zea, M. C. Sexual role identity and anal sex positioning among Brazilian, Colombian, and Dominican immigrant sexual minority men. J. Sex. Res. 59, 632–642 (2022).
Ybarra, M., Price-Feeney, M. & Mwaba, K. Prevalence and correlates of anal sex among secondary school students in Cape Town, South Africa. AIDS Care 30, 821–829 (2018).
Dangerfield, D. T. II, Gravitt, P., Rompalo, A. M., Tai, R. & Lim, S. H. Correlates of anal sex roles among Malay and Chinese MSM in Kuala Lumpur, Malaysia. Int. J. STD AIDS 27, 313–320 (2016).
Hensel, D. J., von Hippel, C. D., Lapage, C. C. & Perkins, R. H. Women’s techniques for pleasure from anal touch: results from a U.S. probability sample of women ages 18–93. PLoS ONE 17, e0268785 (2022).
Geynisman-Tan, J. et al. Anal penetrative intercourse as a risk factor for fecal incontinence. Female Pelvic Med. Reconstr. Surg. 24, 252–255 (2018).
Bailey, J. V., Farquhar, C., Owen, C. & Whittaker, D. Sexual behaviour of lesbians and bisexual women. Sex. Transm. Infect. 79, 147–150 (2003).
Clements-Nolle, K., Marx, R., Guzman, R. & Katz, M. HIV prevalence, risk behaviors, health care use, and mental health status of transgender persons: implications for public health intervention. Am. J. Public Health 91, 915–921 (2001).
Nemoto, T., Operario, D., Keatley, J., Han, L. & Soma, T. HIV risk behaviors among male-to-female transgender persons of color in San Francisco. Am. J. Public Health 94, 1193–1199 (2004).
Bauer, G. R., Travers, R., Scanlon, K. & Coleman, T. A. High heterogeneity of HIV-related sexual risk among transgender people in Ontario, Canada: a province-wide respondent-driven sampling survey. BMC Public Health 12, 292 (2012).
Reyes, A. P., León, N. Y., Frost, E. R. & Harley, V. R. Genetic control of typical and atypical sex development. Nat. Rev. Urol. 20, 434–451 (2023).
Berger, I., Ansara, Y. G. & Riggs, D. Intersex people’s experiences of medical interventions, sex education, and physical intimacy. Psychol. Sex. https://doi.org/10.1080/19419899.2023.2252446 (2023).
Dodge, B. et al. Sexual behaviors of U.S. men by self-identified sexual orientation: results from the 2012 national survey of sexual health and behavior. J. Sex. Med. 13, 637–649 (2016).
Branfman, J., Stiritz, S. & Anderson, E. Relaxing the straight male anus: decreasing homohysteria around anal eroticism. Sexualities 21, 109–127 (2018).
Phillips, T. R. et al. Oral, vaginal and anal sexual practices among heterosexual males and females attending a sexual health clinic: a cross-sectional survey in Melbourne, Australia. Int. J. Env. Res. Public Health 18, 12668 (2021).
Branson, R. Anal Sex Statistics, 2024. International Union of Sex Workers https://www.iusw.org/anal-sex-statistics/ (2024).
Goldstein, E. How often Americans have anal sex. Future Method https://futuremethod.com/blogs/the-science-of-sex/statistics-on-american-anal-sex-habits (2019).
Williams, D. J., Coto, L. & Berkowitz, D. “It’s absolutely intense, and I love it!” a qualitative investigation of “pegging” as leisure. Leisure Sci. https://doi.org/10.1080/01490400.2023.2226669 (2023).
Marshall, D. C. et al. Female erectile tissues and sexual dysfunction after pelvic radiotherapy: a scoping review. CA Cancer J. Clin. 21, 353–359 (2022).
Dickstein, D. R. & Marshall, D. C. Top, bottom or vers? Creating a more equitable health system for sexual and gender minority patients with prostate cancer. Nat. Rev. Urol. 19, 321–322 (2022).
Wallington, D. G. & Holliday, E. B. Preparing patients for sexual dysfunction after radiation for anorectal cancers: a systematic review. Pract. Radiat. Oncol. 11, 193–201 (2021).
Perez de Arce, E., Quera, R., Ribeiro Barros, J. & Yukie Sassaki, L. Sexual dysfunction in inflammatory bowel disease: what the specialist should know and ask. Int. J. Gen. Med. 14, 2003–2015 (2021).
Romano, L. et al. Erectile and sexual dysfunction in male and female patients with celiac disease: a cross-sectional observational study. Andrology 10, 910–918 (2022).
Shafik, A. & El-Sibai, O. The anocavernosal erectile dysfunction syndrome. II Anal fissure and erectile dysfunction. Int. J. Impot. Res. 12, 279–283 (2000).
Keller, J. J. & Lin, H. C. Haemorrhoids are associated with erectile dysfunction: a population-based study. Int. J. Androl. 35, 867–872 (2012).
Averyt, J. C. & Nishimoto, P. W. Addressing sexual dysfunction in colorectal cancer survivorship care. J. Gastrointest. Oncol. 5, 388–394 (2014).
Joseph, K. et al. Patient reported quality of life after helical IMRT based concurrent chemoradiation of locally advanced anal cancer. Radiother. Oncol. 120, 228–233 (2016).
Gana, T. & Hunt, L. M. Young women and anal sex. BMJ 378, o1975 (2022).
Chen, E., Dickstein, D. R., Kim, U., Zaorsky, N. & Sanghvi, P. Inclusion of sexual orientation and gender identity in clinical trials is necessary for health equity. Int. J. Radiat. Oncol. Biol. Phys. 116, 118–121 (2023).
Mendez, L. C., Hsieh, E., Earle, C. C. & Wong, S. Synchronous anal canal carcinoma in a heterosexual couple. BMC Cancer 18, 884 (2018).
Benson, A. B. et al. Anal carcinoma, version 2.2023, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 21, 653–677 (2023).
Wheldon, C. W., Sykes, K. J., Ramaswamy, M., Bass, S. B. & Collins, B. N. Integrating HPV vaccination within prep care delivery for underserved populations: a mixed methods feasibility study. J. Commun. Health 48, 640–651 (2023).
Romano, L. et al. Sexual dysfunction in gastroenterological patients: do gastroenterologists care enough? A nationwide survey from the Italian Society of Gastroenterology (SIGE). Dig. Liver Dis. 54, 1494–1501 (2022).
Liot, E. et al. Patients’ related sexual outcomes in colorectal surgery. Front. Oncol. 12, 968978 (2022).
Mercer, C. H. et al. Changes in sexual attitudes and lifestyles in Britain through the life course and over time: findings from the National Surveys of Sexual Attitudes and Lifestyles (Natsal). Lancet 382, 1781–1794 (2013).
Rissel, C. et al. Heterosexual experience and recent heterosexual encounters among Australian adults: the Second Australian Study of Health and Relationships. Sex. Health 11, 416–426 (2014).
de Visser, R. O., Smith, A. M., Rissel, C. E., Richters, J. & Grulich, A. E. Sex in Australia: heterosexual experience and recent heterosexual encounters among a representative sample of adults. Aust. N. Z. J. Public. Health 27, 146–154 (2003).
Ipsos. LGBT+ Pride 2021 Global Survey. Ipsos https://www.ipsos.com/sites/default/files/ct/news/documents/2021-06/lgbt-pride-2021-global-survey-ipsos.pdf (2021).
Ipsos. LGBT+ Pride 2023. Ipsos https://www.ipsos.com/sites/default/files/ct/news/documents/2023-05/Ipsos%20LGBT%2B%20Pride%202023%20Global%20Survey%20Report%20-%20rev.pdf (2023).
Jones, J. M. U.S. LGBT Identification Steady at 7.2%. Gallup https://news.gallup.com/poll/470708/lgbt-identification-steady.aspx#:~:Text=Story%20Highlights&Text=Learn%20more%20in%20Gallup’;s%202024,in%202022%2C%20at%207.2%25 (2023).
Buston, K. & Hart, G. Heterosexism and homophobia in Scottish school sex education: exploring the nature of the problem. J. Adolesc. 24, 95–109 (2001).
Hollows, K. Anodyspareunia: a novel sexual dysfunction? An exploration into anal sexuality. Sex. Relatsh Ther. 22, 429–443 (2007).
Kutner, B. A., Simoni, J. M., Aunon, F. M., Creegan, E. & Balán, I. C. How stigma toward anal sexuality promotes concealment and impedes health-seeking behavior in the U.S. among cisgender men who have sex with men. Arch. Sex. Behav. 50, 1651–1663 (2021).
Parent, M. C. & Wille, L. Heterosexual self-presentation, identity management, and sexual functioning among men who have sex with men. Arch. Sex. Behav. 50, 3155–3162 (2021).
Crowhurst, M. Heteroprivilegism: three layers of discriminatory practices that target non-heterosexual subjects. NJ 26, 21–34 (2002).
Quatrini, A. On social injustice and word creation on the web, 2020 BLM movement in relation to neologisms. Eur. Acad. Res. 9, 3355 (2021).
Worthen, M. G. F. Transgender under fire: hetero-cis-normativity and military students’ attitudes toward trans issues and trans service members post DADT. Sex. Res. Soc. Policy 16, 289–308 (2019).
Winder, T. J. A. The discursive work of “Bottom-Shaming”: sexual positioning discourse in the construction of black masculinity. Gend. Soc. 37, 774–799 (2023).
Gruskin, S., Yadav, V., Castellanos-Usigli, A., Khizanishvili, G. & Kismödi, E. Sexual health, sexual rights and sexual pleasure: meaningfully engaging the perfect triangle. Sex. Reprod. Health Matters 27, 29–40 (2019).
Hoare, B. S. & Khan, Y. S. Anatomy, abdomen and pelvis: female internal genitals. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK554601/ (updated 24 July 2023).
Gaither, T. W. et al. Atlas of the receptive anal sex experience among people with prostates. J. Sex. Med. 20, 126–138 (2023).
Everaert, K. et al. Neuroanatomy and neurophysiology related to sexual dysfunction in male neurogenic patients with lesions to the spinal cord or peripheral nerves. Spinal Cord 48, 182–191 (2010).
Everaerd, W. in The International Encyclopedia of Human Sexuality (eds Bolin, A. Whelehan, P.) 1115–1354 (Wiley, 2015).
Masters, W. & Johnson, V. E. Human Sexual Response (Ishi Press International, 2010).
IsHak, W. W. The Textbook of Clinical Sexual Medicine 1st edn (Springer International Publishing, 2017).
Burnett, A. L. The role of nitric oxide in erectile dysfunction: implications for medical therapy. J. Clin. Hypertens. 8, 53–62 (2006).
Krassioukov, A. & Elliott, S. Neural control and physiology of sexual function: effect of spinal cord injury. Top. Spinal Cord Inj. Rehabil. 23, 1–10 (2017).
Nummenmaa, L., Suvilehto, J. T., Glerean, E., Santtila, P. & Hietanen, J. K. Topography of human erogenous zones. Arch. Sex. Behav. 45, 1207–1216 (2016).
Ahmed, A., Arbor, T. C. & Qureshi, W. A. Anatomy, abdomen and pelvis: anal canal. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK554531/ (updated 22 May 2023).
Shah, A. & Khan, Y. S. Anatomy, abdomen and pelvis: arteries and veins. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK560486/ (updated 25 July 2023).
Yeoh, S. et al. Incorporating blood flow in nerve injury and regeneration assessment. Front. Surg. 9, 862478 (2022).
Oakley, S. H. et al. Innervation and histology of the clitoral–urethal complex: a cross-sectional cadaver study. J. Sex. Med. 10, 2211–2218 (2013).
Agnew, J. Some anatomical and physiological aspects of anal sexual practices. J. Homosex. 12, 75–96 (1985).
Gorsch, R. V. Proctologic Anatomy (Williams & Wilkins, 1955).
Marshall, D. C. et al. A first radiotherapy application of functional bulboclitoris anatomy, a novel female sexual organ-at-risk, and organ-sparing feasibility study. Br. J. Radiol. 94, 20201139 (2021).
Previnaire, J. G. The importance of the bulbocavernosus reflex. Spinal Cord Ser. Cases 4, 2 (2018).
Niu, X. et al. Application of bulbocavernosus reflex combined with anal sphincter electromyography in the diagnosis of MSA and PD. Int. J. Neurosci. 132, 851–856 (2022).
Panchatsharam, P. K., Durland, J. & Zito, P. M. Physiology, erection. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK513278/ (updated 1 May 2023).
Woodard, T. L. & Diamond, M. P. Physiologic measures of sexual function in women: a review. Fertil. Steril. 92, 19–34 (2009).
Cunningham, D. J. Cunningham’s Textbook of Anatomy 11th edn (ed. Romanes, G. J.) (Oxford University Press, 1972).
Goligher, J. C., Duhle, H. L. & Nixon, H. H. Surgery of The Anus, Rectum and Colon 4th edn (Bailliere Tindale, 1981).
Kinsey, A. C., Pomery, W. B. & Martin, C. E. Sexual Behavior in the Human Male (W.B. Saunders Co., 1948).
Fowler, C. J., Swinn, M. J., Goodwin, R. J., Oliver, S. & Craggs, M. Studies of the latency of pelvic floor contraction during peripheral nerve evaluation show that the muscle response is reflexly mediated. J. Urol. 163, 881–883 (2000).
Previnaire, J. G. & Alexander, M. The sacral exam — what is needed to best care for our patients? Spinal Cord. Ser. Cases 6, 3 (2020).
Bharucha, A. E. Pelvic floor: anatomy and function. Neurogastroenterol. Motil. 18, 507–519 (2006).
Mercado-Perez, A. & Beyder, A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 19, 283–296 (2022).
Furness, J. B., Rivera, L. R., Cho, H. J., Bravo, D. M. & Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10, 729–740 (2013).
Gilbert, J. A. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Dai, Z., Wu, Z., Hang, S., Zhu, W. & Wu, G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Mol. Hum. Reprod. 21, 389–409 (2015).
Palumbo, V. D. et al. Altered gut microbic flora and haemorrhoids: could they have a possible relationship? J. Clin. Med. 12, 2198 (2023).
Priadko, K. et al. Intestinal microbiota, intestinal permeability and the urogenital tract: is there a pathophysiological link? J. Physiol. Pharmacol. 73, 575–585 (2022).
Cai, P., Rong, H., Zhu, Q., Dai, X. & Zhao, J. The potential roles of gut microbiome in anal fistula. AMB Express 13, 58 (2023).
Li, G. et al. Differences in the gut microbiome of women with and without hypoactive sexual desire disorder: case control study. J. Med. Internet Res. 23, e25342 (2021).
Geng, Q. et al. Correlation between gut microbiota diversity and psychogenic erectile dysfunction. Transl. Androl. Urol. 10, 4412–4421 (2021).
Eltas, A., Oguz, F., Uslu, M. O. & Akdemir, E. The effect of periodontal treatment in improving erectile dysfunction: a randomized controlled trial. J. Clin. Periodontol. 40, 148–154 (2013).
Goh, C. E. et al. Association between nitrate-reducing oral bacteria and cardiometabolic outcomes: results from ORIGINS. J. Am. Heart Assoc. 8, e013324 (2019).
Gualtero, D. F. et al. Oral microbiome mediated inflammation, a potential inductor of vascular diseases: a comprehensive review. Front. Cardiovasc. Med. 10, 1250263 (2023).
Vujkovic-Cvijin, I. et al. HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun. 11, 2448 (2020).
Carda-Diéguez, M. et al. Variations in vaginal, penile, and oral microbiota after sexual intercourse: a case report. Front. Med. 6, 178 (2019).
Zabor, E. C. et al. Association between periodontal disease, bacterial vaginosis, and sexual risk behaviours. J. Clin. Periodontol. 37, 888–893 (2010).
Li, S. et al. Alteration in oral microbiome among men who have sex with men with acute and chronic HIV infection on antiretroviral therapy. Front. Cell Infect. Microbiol. 11, 695515 (2021).
Amato, K. R. et al. The human gut microbiome and health inequities. Proc. Natl Acad. Sci. USA 118, e2017947118 (2021).
Ursell, L. K. et al. The interpersonal and intrapersonal diversity of human-associated microbiota in key body sites. J. Allergy Clin. Immunol. 129, 1204–1208 (2012).
Iljazovic, A., Amend, L., Galvez, E. J. C., de Oliveira, R. & Strowig, T. Modulation of inflammatory responses by gastrointestinal Prevotella spp. – from associations to functional studies. Int. J. Med. Microbiol. 311, 151472 (2021).
Gorvitovskaia, A., Holmes, S. P. & Huse, S. M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 4, 15 (2016).
Vélez, C., Casimiro, I., Pitts, R., Streed, C. Jr. & Paul, S. Digestive health in sexual and gender minority populations. Am. J. Gastroenterol. 117, 865–875 (2022).
Hjorth, M. F. et al. Prevotella-to-Bacteroides ratio predicts body weight and fat loss success on 24-week diets varying in macronutrient composition and dietary fiber: results from a post-hoc analysis. Int. J. Obes. 43, 149–157 (2019).
Altmäe, S., Franasiak, J. M. & Mändar, R. The seminal microbiome in health and disease. Nat. Rev. Urol. 16, 703–721 (2019).
Kelley, C. F. et al. Condomless receptive anal intercourse is associated with markers of mucosal inflammation in a cohort of men who have sex with men in Atlanta, Georgia. J. Int. AIDS Soc. 24, e25859 (2021).
Javanbakht, M., Stahlman, S., Pickett, J., LeBlanc, M.-A. & Gorbach, P. M. Prevalence and types of rectal douches used for anal intercourse: results from an international survey. BMC Infect. Dis. 14, 95 (2014).
Haaland, R. E. et al. Repeated rectal application of a hyperosmolar lubricant is associated with microbiota shifts but does not affect PrEP drug concentrations: results from a randomized trial in men who have sex with men. J. Int. AIDS Soc. 21, e25199 (2018).
Dong, T. S. et al. How discrimination gets under the skin: biological determinants of discrimination associated with dysregulation of the brain-gut microbiome system and psychological symptoms. Biol. Psychiatry 94, 203–214 (2023).
Mallott, E. K. et al. Human microbiome variation associated with race and ethnicity emerges as early as 3 months of age. PLoS Biol. 21, e3002230 (2023).
Madison, A. & Kiecolt-Glaser, J. K. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 28, 105–110 (2019).
McCabe, M. P. et al. Incidence and prevalence of sexual dysfunction in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J. Sex. Med. 13, 144–152 (2016).
Faubion, S. S. & Rullo, J. E. Sexual dysfunction in women: a practical approach. Am. Fam. Physician 92, 281–288 (2015).
Phillips, N. A. Female sexual dysfunction: evaluation and treatment. Am. Fam. Physician 62, 127–136, 141-122 (2000).
Reed, G. M. et al. Disorders related to sexuality and gender identity in the ICD-11: revising the ICD-10 classification based on current scientific evidence, best clinical practices, and human rights considerations. World Psychiatry 15, 205–221 (2016).
Rosser, B. R., Metz, M. E., Bockting, W. O. & Buroker, T. Sexual difficulties, concerns, and satisfaction in homosexual men: an empirical study with implications for HIV prevention. J. Sex Marital Ther. 23, 61–73 (1997).
Damon, W. & Rosser, B. R. Anodyspareunia in men who have sex with men: prevalence, predictors, consequences and the development of DSM diagnostic criteria. J. Sex Marital Ther. 31, 129–141 (2005).
Rosser, B. R., Short, B. J., Thurmes, P. J. & Coleman, E. Anodyspareunia, the unacknowledged sexual dysfunction: a validation study of painful receptive anal intercourse and its psychosexual concomitants in homosexual men. J. Sex Marital Ther. 24, 281–292 (1998).
Plewka, A. & Haak, D. Anal dyspareunia — biological and psychosocial correlates of painful anal intercourses in population. Psychiatr. Pol. 57, 457–465 (2023).
Peixoto, M. M. & Nobre, P. Prevalence of sexual problems and associated distress among gay and heterosexual men. Sex. Relatsh Ther. 30, 211–225 (2015).
Peixoto, M. M. & Nobre, P. Prevalence of sexual problems and associated distress among lesbian and heterosexual women. J. Sex Marital Ther. 41, 427–439 (2015).
Gaither, T. W. et al. Relationship between pelvic sensations and lifetime exposure to receptive anal intercourse among people with prostates. J. Sex. Med. 20, 1195–1205 (2023).
Grabski, B. & Kasparek, K. Sexual anal pain in gay and bisexual men: in search of explanatory factors. J. Sex. Med. 17, 716–730 (2020).
Cheng, P. J. Sexual dysfunction in men who have sex with men. Sex. Med. Rev. 10, 130–141 (2022).
Vansintejan, J., Vandevoorde, J. & Devroey, D. The GAy MEn sex studies: anodyspareunia among Belgian gay men. Sex. Med. 1, 87–94 (2013).
Nercessian, T.-R., Banbury, S. & Chandler, C. A systematic review looking at anodyspareunia among cisgender men and women. J. Sex. Marital Ther. 49, 829–841 (2023).
Yessick, L. R., Gauvin, S. E. M., Salomons, T. V. & Pukall, C. F. Pain characteristics, sexual script flexibility, and penetration control cognitions in those experiencing anodyspareunia. Psychol. Sex. 14, 321–336 (2023).
Shah, S. G. et al. Effect of magnetic endoscope imaging on patient tolerance and sedation requirements during colonoscopy: a randomized controlled trial. Gastrointest. Endosc. 55, 832–837 (2002).
Marchi, M. et al. Post-traumatic stress disorder among LGBTQ people: a systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 32, e44 (2023).
Christian, L. M. et al. A biopsychosocial framework for understanding sexual and gender minority health: a call for action. Neurosci. Biobehav. Rev. 129, 107–116 (2021).
Jones, J. F. X. in Primer on the Autonomic Nervous System 3rd edn (eds Robertson, D. et al.) 601–602 (Academic Press, 2012).
Mathias, C. J. & Low, D. A. in Textbook of Neural Repair and Rehabilitation: Volume 2: Medical Neurorehabilitation (eds Gert, K. et al.) 415–436 (Cambridge University Press, 2014).
El Muhtaseb, M. S. et al. Depression and anxiety among chronic anal fissure patients. Int. J. Surg. Open 46, 100518 (2022).
Rosa, L. L. Anodyspareunia. Anal pain during anoreceptive sex in men who have sex with men [Spanish]. Rev. Argent. Coloproctología 31, 8–20 (2020).
Wheldon, C. W. et al. Pain and loss of pleasure in receptive anal sex for gay and bisexual men following prostate cancer treatment: results from the Restore-1 study. J. Sex. Res. 59, 826–833 (2022).
Chen, T.-C. et al. Clinical characteristics and treatment outcome of oropharyngeal squamous cell carcinoma in an endemic betel quid region. Sci. Rep. 10, 526 (2020).
Schönhofen, J. et al. Incidental findings during computed tomographic angiography diagnostic work-up in patients with arteriogenic erectile dysfunction. Swiss Med. Wkly 149, w20154 (2019).
Chadwick, S. B., Francisco, M. & van Anders, S. M. When orgasms do not equal pleasure: accounts of “bad” orgasm experiences during consensual sexual encounters. Arch. Sex. Behav. 48, 2435–2459 (2019).
Sartori, D. V. B. et al. Pelvic floor muscle strength is correlated with sexual function. Investig. Clin. Urol. 62, 79–84 (2021).
Graber, B. & Kline-Graber, G. Female orgasm: role of pubococcygeus muscle. J. Clin. Psychiatry 40, 348–351 (1979).
Shafik, A. The role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int. Urogynecol. J. Pelvic Floor Dysfunct. 11, 361–376 (2000).
Padoa, A., McLean, L., Morin, M. & Vandyken, C. “The overactive pelvic floor (OPF) and sexual dysfunction” Part 1: pathophysiology of OPF and its impact on the sexual response. Sex. Med. Rev. 9, 64–75 (2021).
Padoa, A., McLean, L., Morin, M. & Vandyken, C. The overactive pelvic floor (OPF) and sexual dysfunction. Part 2: evaluation and treatment of sexual dysfunction in OPF patients. Sex. Med. Rev. 9, 76–92 (2021).
Turnbull, G. B. Sexual counseling: the forgotten aspect of ostomy rehabilitation. J. Sex. Educ. Ther. 26, 189–195 (2001).
Li, C.-C. Sexuality among patients with a colostomy: an exploration of the influences of gender, sexual orientation, and Asian heritage. J. Wound Ostomy Cont. Nurs. 36, 288–296 (2009).
Graziottin, A. & Serafini, A. HPV infection in women: psychosexual impact of genital warts and intraepithelial lesions. J. Sex. Med. 6, 633–645 (2009).
Cosnes, J., Gower-Rousseau, C., Seksik, P. & Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140, 1785–1794 (2011).
Elfeki, H. et al. Patient and healthcare professional perceptions of colostomy-related problems and their impact on quality of life following rectal cancer surgery. BJS Open 2, 336–344 (2018).
Lemini, R. et al. Permanent stoma: a quality outcome in treatment of rectal cancer and its impact on length of stay. BMC Surg. 21, 163 (2021).
Glynne-Jones, R. et al. Tumour- and treatment-related colostomy rates following mitomycin C or cisplatin chemoradiation with or without maintenance chemotherapy in squamous cell carcinoma of the anus in the ACT II trial. Ann. Oncol. 25, 1616–1622 (2014).
Dames, N. B. et al. ‘Let’s talk about sex’: a patient-led survey on sexual function after colorectal and pelvic floor surgery. Colorectal Dis. 23, 1524–1551 (2021).
Reese, J. B. et al. Gastrointestinal ostomies and sexual outcomes: a comparison of colorectal cancer patients by ostomy status. Support. Care Cancer 22, 461–468 (2014).
Kibret, A. A., Oumer, M. & Moges, A. M. Prevalence and associated factors of hemorrhoids among adult patients visiting the surgical outpatient department in the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS ONE 16, e0249736 (2021).
Randhawa, N. et al. Endoscopic ultrasound-guided botox injection for refractory anal fissure. J. Clin. Med. 11, 6207 (2022).
Aguilar-Alvarado, M. Y., Baker, B., Chiu, L. S. & Shah, M. K. Benign colorectal disorders. Prim. Care 50, 461–480 (2023).
Lundell, L. Reflux symptoms: functional and structural diseases and the approach from the GI specialist. Dig. Dis. 39, 590–597 (2021).
Goddard, S. L. et al. Self-reported anal symptoms and their association with anal pathology among gay and bisexual men: a cross-sectional observational analysis. Sex. Health 18, 123–129 (2021).
Goldstone, S. E. The Ins and Outs of Gay Sex: A Medical Handbook for Men (Dell Publishing, 1999).
Stratton, N. L. When the “Oohs” Are Painful, Not Pleasurable: An Investigation of Anodyspareunia Among Gay, Bisexual, and Queer Men. PhD Thesis, Ryerson University (2019).
van Reijn-Baggen, D. A., Elzevier, H. W., Putter, H., Pelger, R. C. M. & Han-Geurts, I. J. M. Pelvic floor physical therapy in patients with chronic anal fissure: a randomized controlled trial. Tech. Coloproctol. 26, 571–582 (2022).
Abdelaziz, A. S., Ghoneem, A. M. & Elewesy, E. A. The impact of surgical hemorrhoidectomy on male sexual function: a preliminary study. Urol. Ann. 11, 235–240 (2019).
Gao, D. J., Guo, Y. S., Yu, H. Y., Wang, Y. J. & Cui, W. G. Prevalence and related factors of prostatitis-like symptoms in young men [Chinese]. Zhonghua Nan Ke Xue 13, 1087–1090 (2007).
Lee Mortensen, G. & Larsen, H. K. Quality of life of homosexual males with genital warts: a qualitative study. BMC Res. Notes 3, 280 (2010).
Camargo, C. C., D’Elia, M. P. B. & Miot, H. A. Quality of life in men diagnosed with anogenital warts. Bras. Dermatol. 92, 427–429 (2017).
Sperber, A. D. & Dekel, R. Irritable bowel syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syndromes. J. Neurogastroenterol. Motil. 16, 113–119 (2010).
Fass, R., Fullerton, S., Naliboff, B., Hirsh, T. & Mayer, E. A. Sexual dysfunction in patients with irritable bowel syndrome and non-ulcer dyspepsia. Digestion 59, 79–85 (1998).
Gros, M., Gros, B., Mesonero, J. E. & Latorre, E. Neurotransmitter dysfunction in irritable bowel syndrome: emerging approaches for management. J. Clin. Med. 10, 3429 (2021).
Vahora, I. S., Tsouklidis, N., Kumar, R., Soni, R. & Khan, S. How serotonin level fluctuation affects the effectiveness of treatment in irritable bowel syndrome. Cureus 12, e9871 (2020).
Spohn, S. N. & Mawe, G. M. Non-conventional features of peripheral serotonin signalling — the gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 14, 412–420 (2017).
Staudacher, H. M., Black, C. J., Teasdale, S. B., Mikocka-Walus, A. & Keefer, L. Irritable bowel syndrome and mental health comorbidity — approach to multidisciplinary management. Nat. Rev. Gastroenterol. Hepatol. 20, 582–596 (2023).
Zamani, M., Alizadeh-Tabari, S. & Zamani, V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 50, 132–143 (2019).
Jing, E. & Straw-Wilson, K. Sexual dysfunction in selective serotonin reuptake inhibitors (SSRIs) and potential solutions: a narrative literature review. Ment. Health Clin. 6, 191–196 (2016).
Bradford, K. et al. Association between early adverse life events and irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 10, 385–390.e1-3 (2012).
Jagielski, C. H., Chey, W. D. & Riehl, M. E. Influence of trauma on clinical outcomes, quality of life and healthcare resource utilization following psychogastroenterology intervention. J. Psychosom. Res. 146, 110481 (2021).
Marshall, D. C. et al. Effects of trauma history on cancer-related screening, diagnosis, and treatment. Lancet Oncol. 24, e426–e437 (2023).
Stephenson, R. & Finneran, C. Receipt and perpetration of intimate partner violence and condomless anal intercourse among gay and bisexual men in Atlanta. AIDS Behav. 21, 2253–2260 (2017).
Storholm, E. D. et al. Intimate partner violence and the sexual health of sexual minority men. LGBT Health 10, S39–S48 (2023).
Campbell, J. et al. Intimate partner violence and physical health consequences. Arch. Intern. Med. 162, 1157–1163 (2002).
Calton, J. M., Cattaneo, L. B. & Gebhard, K. T. Barriers to help seeking for lesbian, gay, bisexual, transgender, and queer survivors of intimate partner violence. Trauma Violence Abuse 17, 585–600 (2016).
Huecker, M. R., King, K. C., Jordan, G. A. & Smock, W. Domestic Violence. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK499891/ (updated 9 Apr 2023).
Granot, M. et al. Trauma, attachment style, and somatization: a study of women with dyspareunia and women survivors of sexual abuse. BMC Womens Health 18, 29 (2018).
Postma, R., Bicanic, I., van der Vaart, H. & Laan, E. Pelvic floor muscle problems mediate sexual problems in young adult rape victims. J. Sex. Med. 10, 1978–1987 (2013).
Kombe Kombe, A. J. et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front. Public Health 8, 552028 (2020).
Pennycook, K. B. & McCready, T. A. Condyloma acuminata. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK547667/ (updated 21 Jun 2023).
Leslie, S. W., Sajjad, H. & Kumar, S. Genital warts. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK441884/ (updated 30 May 2023).
Tyros, G., Mastraftsi, S., Gregoriou, S. & Nicolaidou, E. Incidence of anogenital warts: epidemiological risk factors and real-life impact of human papillomavirus vaccination. Int. J. STD AIDS 32, 4–13 (2021).
Jin, F. et al. Risk factors for genital and anal warts in a prospective cohort of HIV-negative homosexual men: the HIM study. Sex. Transm. Dis. 34, 488–493 (2007).
Vela, S. et al. Effectiveness of physically ablative and pharmacological treatments for anal condyloma in HIV-infected men. PLoS ONE 13, e0199033 (2018).
Irisawa, R., Tsuboi, R., Saito, M. & Harada, K. Treatment of intra-anal warts with imiquimod 5% cream: a single-center prospective open study. J. Dermatol. 48, 476–480 (2021).
Luz, I. et al. High prevalence of anal sexually transmitted infections among men who have sex with men and transgender women attending a clinic for prevention of anal cancer in Salvador, Brazil. Pathogens 12, 1297 (2023).
Davis, T. W. & Goldstone, S. E. Sexually transmitted infections as a cause of proctitis in men who have sex with men. Dis. Colon Rectum 52, 507–512 (2009).
Martin, T. et al. Receptive anal intercourse in patients with inflammatory bowel disease: a clinical review. Inflamm. Bowel Dis. 23, 1285–1292 (2017).
Hirshfield, S. et al. Sexual dysfunction in an Internet sample of U.S. men who have sex with men. J. Sex. Med. 7, 3104–3114 (2010).
Zucker, R. et al. Triple site sexually transmitted infection testing as a crucial component of surveillance for men who have sex with men: a prospective cohort study. Int. J. STD AIDS 33, 114–122 (2022).
De Vincentis, S., Tartaro, G., Rochira, V. & Santi, D. HIV and sexual dysfunction in men. J. Clin. Med. 10, 1088 (2021).
Grov, C., Westmoreland, D., Carneiro, P. B., Bauermeister, J. A. & Carrico, A. W. Getting clear about rectal douching among men who have sex with men. Arch. Sex. Behav. 50, 2911–2920 (2021).
Clay, P. G. & Crutchley, R. D. Noninfectious diarrhea in HIV seropositive individuals: a review of prevalence rates, etiology, and management in the era of combination antiretroviral therapy. Infect. Dis. Ther. 3, 103–122 (2014).
Nimbi, F. M. et al. Sexual desire and fantasies in the LGBT+ community: focus on lesbian women and gay men. Curr. Sex. Health Rep. 12, 153–161 (2020).
Vansintejan, J., Janssen, J., Van De Vijver, E., Vandevoorde, J. & Devroey, D. The gay men sex studies: prevalence of sexual dysfunctions in Belgian HIV+ gay men. HIV AIDS 5, 89–96 (2013).
Jagdish, R. K. Sexual dysfunctions and their treatment in liver diseases. World J. Hepatol. 14, 1530–1540 (2022).
Romano, L. et al. Sexual dysfunction in patients with chronic gastrointestinal and liver diseases: a neglected issue. Sex. Med. Rev. 10, 620–631 (2022).
Gorgos, L. Sexual transmission of viral hepatitis. Infect. Dis. Clin. North Am. 27, 811–836 (2013).
Cota, J. M. et al. High frequency of potential phosphodiesterase type 5 inhibitor drug interactions in males with HIV infection and erectile dysfunction. PLoS ONE 16, e0250607 (2021).
Rangnekar, A. S. & Fontana, R. J. Managing drug-drug interactions with boceprevir and telaprevir. Clin. Liver Dis. 1, 36–40 (2012).
Burger, D. et al. Clinical management of drug–drug interactions in HCV therapy: challenges and solutions. J. Hepatol. 58, 792–800 (2013).
Fernández-Huerta, M. et al. Sexual transmission of intestinal parasites and other enteric pathogens among men who have sex with men presenting gastrointestinal symptoms in an STI unit in Barcelona, Spain: a cross-sectional study. Am. J. Trop. Med. Hyg. 101, 1388–1391 (2019).
Reses, H. E. et al. Risk factors for sporadic Giardia infection in the USA: a case-control study in Colorado and Minnesota. Epidemiol. Infect. 146, 1071–1078 (2018).
Sewell, K. K., McGarrity, L. A. & Strassberg, D. S. Sexual behavior, definitions of sex, and the role of self-partner context among lesbian, gay, and bisexual adults. J. Sex. Res. 54, 825–831 (2017).
Conners, E. E., Miller, A. D., Balachandran, N., Robinson, B. M. & Benedict, K. M. Giardiasis outbreaks — United States, 2012-2017. MMWR Morb. Mortal. Wkly Rep. 70, 304–307 (2021).
Heller, L. The Human Rights to Water and Sanitation (Cambridge University Press, 2022).
Blackburn, A. M., Katz, B. W., Oesterle, D. W. & Orchowski, L. M. Preventing sexual violence in sexual orientation and gender diverse communities: a call to action. Eur. J. Psychotraumatol. 15, 2297544 (2023).
Porta, C. M. et al. “Kicked out”: LGBTQ youths’ bathroom experiences and preferences. J. Adolesc. 56, 107–112 (2017).
Morin, J. Anal Pleasure & Health: A Guide For Men, Women, and Couples 4th edn (Down There Press, 2010).
GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 5, 17–30 (2020).
Bisgaard, T. H., Allin, K. H., Keefer, L., Ananthakrishnan, A. N. & Jess, T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 19, 717–726 (2022).
McDowell, C., Farooq, U. & Haseeb, M. Inflammatory bowel disease. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK470312/ (updated 4 Aug 2023).
Uhlir, V., Stallmach, A. & Grunert, P. C. Fatigue in patients with inflammatory bowel disease — strongly influenced by depression and not identifiable through laboratory testing: a cross-sectional survey study. BMC Gastroenterol. 23, 288 (2023).
Balestrieri, P. et al. Nutritional aspects in inflammatory bowel diseases. Nutrients 12, 372 (2020).
Adolph, T. E. et al. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 19, 753–767 (2022).
Kamp, K., Dudley-Brown, S., Heitkemper, M., Wyatt, G. & Given, B. Symptoms among emerging adults with inflammatory bowel disease: a descriptive study. Res. Nurs. Health 43, 48–55 (2020).
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156, 1345–1353.e4 (2019).
Dibley, L., Norton, C., Schaub, J. & Bassett, P. Experiences of gay and lesbian patients with inflammatory bowel disease: a mixed methods study. Gastrointest. Nurs. 12, 19–30 (2014).
Ghusn, W. et al. S999. Social determinants of health in LGBTQIA+ patients with inflammatory bowel disease. Am. J. Gastroenterol. 117, e725 (2022).
Panés, J. & Rimola, J. Perianal fistulizing Crohn’s disease: pathogenesis, diagnosis and therapy. Nat. Rev. Gastroenterol. Hepatol. 14, 652–664 (2017).
de Zeeuw, S. et al. Update of complications and functional outcome of the ileo-pouch anal anastomosis: overview of evidence and meta-analysis of 96 observational studies. Int. J. Colorectal Dis. 27, 843–853 (2012).
Heimann, T. M., Swaminathan, S., Slater, G. I. & Kurtz, R. J. Perianal fistula after ileoanal pouch in patients with ulcerative colitis: a review of 475 patients operated on at a major IBD center. Dis. Colon Rectum 65, 76–82 (2022).
Gomez, J. et al. Hidradenitis suppurativa in sexual and gender minorities: a review and considerations for providers. J. Am. Acad. Dermatol. 89, 795–801 (2023).
Roda, G. et al. Systematic review with meta-analysis: proximal disease extension in limited ulcerative colitis. Aliment. Pharmacol. Ther. 45, 1481–1492 (2017).
Schlegel, N. et al. Sphincter-sparing intersphincteric rectal resection as an alternative to proctectomy in long-standing fistulizing and stenotic Crohn’s proctitis? Int. J. Colorectal Dis. 30, 655–663 (2015).
Park, S. J., Kim, W. H. & Cheon, J. H. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J. Gastroenterol. 20, 11525–11537 (2014).
Chun, A. B., Rose, S., Mitrani, C., Silvestre, A. J. & Wald, A. Anal sphincter structure and function in homosexual males engaging in anoreceptive intercourse. Am. J. Gastroenterol. 92, 465–468 (1997).
Lin, X. et al. Intestinal strictures in Crohn’s disease: a 2021 update. Ther. Adv. Gastroenterol. 15, 17562848221104951 (2022).
Lightner, A. L. & Regueiro, M. Anorectal strictures in complex perianal CD: how to approach. Clin. Colon Rectal Surg. 35, 44–50 (2022).
Pogacnik, J. S. & Salgado, G. Perianal Crohn’s disease. Clin. Colon Rectal Surg. 32, 377–385 (2019).
Bonheur, J. L., Braunstein, J., Korelitz, B. I. & Panagopoulos, G. Anal skin tags in inflammatory bowel disease: new observations and a clinical review. Inflamm. Bowel Dis. 14, 1236–1239 (2008).
Safar, B. & Sands, D. Perianal Crohn’s disease. Clin. Colon Rectal Surg. 20, 282–293 (2007).
Opar, S. P. Photo quiz. Painful perianal lesions. Am. Fam. Physician 82, 419–421 (2010).
Sommer, K. et al. Intestinal mucosal wound healing and barrier integrity in IBD-crosstalk and trafficking of cellular players. Front. Med. 8, 643973 (2021).
Alexakis, C. et al. Ano-genital granulomatosis and Crohn’s disease: a case series of males presenting with genital lymphoedema. J. Crohns Colitis 11, 454–459 (2016).
Dederichs, F. et al. Genital granulomatosis in male and female patients with Crohn’s disease: clinical presentation and treatment outcomes. J. Crohns Colitis 12, 197–203 (2018).
Crohn’s and Colitis UK. Sex and relationships.Crohn’s & Colitis UK https://crohnsandcolitis.org.uk/media/wrshmklm/sex-and-relationships-ed5-2022.pdf (2022).
Hay, M. I have an inflammatory bowel disease. Here’s how it affects my sex life. Vice https://www.vice.com/en/article/pkpv39/how-inflammatory-bowel-disease-ulcerative-colitis-affects-my-sex-life (2022).
SEX: Straight Up (Well, Maybe Not So Straight). The Gay Digest https://gaydigest.wordpress.com/tag/crohns/ (2010).
Rui, W., Zhaoqi, L., Shaojun, L. & Decai, Z. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: a systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 13, e065186 (2023).
Marín, L. et al. Sexual function and patients’ perceptions in inflammatory bowel disease: a case-control survey. J. Gastroenterol. 48, 713–720 (2013).
Zamponi, V. et al. Female sexual dysfunction in primary adrenal insufficiency. J. Clin. Med. 10, 2767 (2021).
Beese, S. E., Harris, I. M., Dretzke, J. & Moore, D. Body image dissatisfaction in patients with inflammatory bowel disease: a systematic review. BMJ Open Gastroenterol. 6, e000255 (2019).
Levitan, J., Quinn-Nilas, C., Milhausen, R. & Breuer, R. The relationship between body image and sexual functioning among gay and bisexual men. J. Homosex. 66, 1856–1881 (2019).
Nowicki, G. P., Marchwinski, B. R., O’Flynn, J. L., Griffths, S. & Rodgers, R. F. Body image and associated factors among sexual minority men: a systematic review. Body Image 43, 154–169 (2022).
Khoudari, G. et al. Rates of intestinal resection and colectomy in inflammatory bowel disease patients after initiation of biologics: a cohort study. Clin. Gastroenterol. Hepatol. 20, e974–e983 (2022).
Shen, B. et al. Treatment of pouchitis, Crohn’s disease, cuffitis, and other inflammatory disorders of the pouch: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol. Hepatol. 7, 69–95 (2022).
Scoglio, D., Ahmed Ali, U. & Fichera, A. Surgical treatment of ulcerative colitis: ileorectal vs ileal pouch-anal anastomosis. World J. Gastroenterol. 20, 13211–13218 (2014).
Abdalla, M. et al. Anorectal function after ileo-rectal anastomosis is better than pelvic pouch in selected ulcerative colitis patients. Dig. Dis. Sci. 65, 250–259 (2020).
Panganiban, R. & Clarke, K. S2112: Receptive anal intercourse in IBD patients: impact on treatment of anorectal disease. Am. J. Gastroenterol. https://gi.org/wp-content/uploads/2022/10/Colon-Clinical-Vignettes.pdf (2022).
Georganta, I. et al. The incidence of malignancy in the residual rectum of IBD patients after colectomy: a systematic review and meta-analysis. Tech. Coloproctol. 27, 699–712 (2023).
Stellingwerf, M. E. et al. Knotless seton for perianal fistulas: feasibility and effect on perianal disease activity. Sci. Rep. 10, 16693 (2020).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Maddams, J., Utley, M. & Møller, H. Projections of cancer prevalence in the United Kingdom, 2010-2040. Br. J. Cancer 107, 1195–1202 (2012).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72, 409–436 (2022).
Parry, C., Kent, E. E., Mariotto, A. B., Alfano, C. M. & Rowland, J. H. Cancer survivors: a booming population. Cancer Epidemiol. Biomark. Prev. 20, 1996–2005 (2011).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Swerdlow, A. J. et al. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J. Natl Cancer Inst. 97, 1204–1210 (2005).
Hasle, H. et al. Occurrence of cancer in women with Turner syndrome. Br. J. Cancer 73, 1156–1159 (1996).
Baraibar, I. et al. Sex and gender perspectives in colorectal cancer. ESMO Open 8, 101204 (2023).
Abancens, M., Bustos, V., Harvey, H., McBryan, J. & Harvey, B. J. Sexual dimorphism in colon cancer. Front. Oncol. 10, 607909 (2020).
Boutas, I., Kontogeorgi, A., Dimitrakakis, C. & Kalantaridou, S. N. Soy isoflavones and breast cancer risk: a meta-analysis. In Vivo 36, 556–562 (2022).
Benitez Majano, S. et al. Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol. 20, 74–87 (2019).
Benson, A. B. et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J. Natl Compr. Cancer Netw. 16, 874–901 (2018).
Boehmer, U. et al. Assessing the relationship between symptoms and health care utilization in colorectal cancer survivors of different sexual orientations. Support. Care Cancer 29, 5821–5830 (2021).
Ward, H. Cancer, Sex and Intimacy: The Experiences of Gay, Bisexual, and Queer Men. PhD Thesis, University of East London (2022).
Dickstein, D. R., Chen, E., Zaorsky, N. G., Hoffman, K. & Nguyen, P. Do ask, do tell: improving health outcomes for sexual and gender minorities with cancer. JNCI Cancer Spectr. 7, pkad075 (2023).
Mitteldorf, D. Psychotherapy with gay prostate cancer patients. J. Gay Lesbian Psychother. 9, 57–67 (2005).
Fergus, K. D., Gray, R. E. & Fitch, M. I. Sexual dysfunction and the preservation of manhood: experiences of men with prostate cancer. J. Health Psychol. 7, 303–316 (2002).
Kurdek, L. A. Are gay and lesbian cohabiting couples really different from heterosexual married couples? J. Marriage Fam. 66, 880–900 (2004).
Kurdek, L. A. What do we know about gay and lesbian couples? Curr. Direc. Psychol. Sci. 14, 251–254 (2005).
Kurdek, L. A. Differences between heterosexual-nonparent couples and gay, lesbian, and heterosexual-parent couples. J. Fam. Issues 22, 727–754 (2001).
Lin, C., Franceschi, S. & Clifford, G. M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect. Dis. 18, 198–206 (2018).
Stier, E. A., Chigurupati, N. L. & Fung, L. Prophylactic HPV vaccination and anal cancer. Hum. Vaccin. Immunother. 12, 1348–1351 (2016).
Giannone, G. et al. HPV vaccination and HPV-related malignancies: impact, strategies and optimizations toward global immunization coverage. Cancer Treat. Rev. 111, 102467 (2022).
Barroso, L. F. II, Stier, E. A., Hillman, R. & Palefsky, J. Anal cancer screening and prevention: summary of evidence reviewed for the 2021 centers for disease control and prevention sexually transmitted infection guidelines. Clin. Infect. Dis. 74, S179–S192 (2022).
Islami, F., Ferlay, J., Lortet-Tieulent, J., Bray, F. & Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 46, 924–938 (2017).
Grulich, A. E. et al. The epidemiology of anal cancer. Sex. Health 9, 504–508 (2012).
Machalek, D. A. et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 13, 487–500 (2012).
Silverberg, M. J. et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin. Infect. Dis. 54, 1026–1034 (2012).
Braun, H. et al. Cancer in transgender people: evidence and methodological considerations. Epidemiol. Rev. 39, 93–107 (2017).
Fein, L. A. et al. Low perceived anal cancer risk and screening utilization among high-risk transgender men and women living in an HIV / STI epicenter. AIDS Behav. 25, 2210–2218 (2021).
Saleem, A. M. et al. Risk of anal cancer in a cohort with human papillomavirus-related gynecologic neoplasm. Obstet. Gynecol. 117, 643–649 (2011).
Field, J., Fenton, C., Zhang, L., Brickman, C. & Mahadevan, U. Inflammatory bowel disease and the risk of anal squamous intraepithelial lesions and anal cancer in men who have sex with men. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izad266 (2023).
Wong, S. Y. & Colombel, J. F. Cancer screening in patients with inflammatory bowel disease: don’t forget the anus! Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izad267 (2023).
Deshmukh, A. A., Damgacioglu, H., Georges, D., Sonawane, K. & Clifford, G. M. Human papillomavirus-associated anal cancer incidence and burden among us men, according to sexual orientation, human immunodeficiency virus status, and age. Clin. Infect. Dis. 77, 419–424 (2023).
Mauro, G. P., da Conceição Vasconcelos, K. G. M. & Carvalho, H. A. Quality of life and sexual function of men who have sex with men treated for anal cancer: a prospective trial of a neglected population. J. Sex. Med. 18, 1461–1466 (2021).
Yang, M.-J. et al. Psychosocial characteristics and quality of life among sexual and gender minority patients with cancer. JNCI Cancer Spectr. 18, pkad061 (2023).
Herek, G. M. Legal recognition of same-sex relationships in the United States: a social science perspective. Am. Psychol. 61, 607–621 (2006).
Tchelebi, L. T. et al. Current treatment and future directions in the management of anal cancer. CA Cancer J. Clin. 72, 183–195 (2022).
Sobrado, L. F., Nahas, C. S. R., Marques, C. F. S., Sobrado, C. W. & Nahas, S. C. Pretreatment colostomy in patients with anal squamous cell carcinoma: Risk factors for a permanent stoma. J. Surg. Oncol. 126, 740–747 (2022).
Koerber, S. A. et al. Chemoradiation in female patients with anal cancer: patient-reported outcome of acute and chronic side effects. Tumori 105, 174–180 (2019).
Joseph, K. et al. Long-term patient-reported quality of life of anal cancer survivors treated with intensity modulated radiation therapy and concurrent chemotherapy: results from a prospective phase II trial. Int. J. Radiat. Oncol. Biol. Phys. 117, 434–445 (2023).
Gilbert, A. et al. UK national cohort of anal cancer treated with intensity-modulated radiotherapy: one-year oncological and patient-reported outcomes. Eur. J. Cancer 128, 7–16 (2020).
Giglia, M. D. & Stein, S. L. Overlooked long-term complications of colorectal surgery. Clin. Colon Rectal Surg. 32, 204–211 (2019).
Marinello, F. G. et al. Selective approach for upper rectal cancer treatment: total mesorectal excision and preoperative chemoradiation are seldom necessary. Dis. Colon Rectum 58, 556–565 (2015).
van Heinsbergen, M. et al. Functional bowel complaints and quality of life after surgery for colon cancer: prevalence and predictive factors. Colorectal Dis. 22, 136–145 (2020).
Rooney, M. K. et al. Patient-reported bowel function and bowel-related quality of life after pelvic radiation for rectal adenocarcinoma: the impact of radiation fractionation and surgical resection. Clin. Colorectal Cancer 22, 211–221 (2023).
Hall, E. J. Radiobiology for the radiologist (Harper & Row, 1973).
Lukovic, J. et al. Evaluation of dosimetric predictors of toxicity after IMRT with concurrent chemotherapy for anal cancer. Radiother. Oncol. 178, 109429 (2023).
Corrigan, K. L. et al. Patient-reported outcomes after chemoradiation in patients with anal cancer: a qualitative analysis. Adv. Radiat. Oncol. 7, 100986 (2022).
Alayed, Y. et al. Dosimetric predictors of toxicity and quality of life following prostate stereotactic ablative radiotherapy. Radiother. Oncol. 144, 135–140 (2020).
Jackson, A. et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int. J. Radiat. Oncol. Biol. Phys. 49, 685–698 (2001).
Untiedt, S. et al. Impact of dose escalation on colostomy-free survival and treatment outcome in squamous cell anal carcinoma. Strahlenther. Onkol. 199, 749–760 (2023).
Myerson, R. J. et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int. J. Radiat. Oncol. Biol. Phys. 74, 824–830 (2009).
Da Silva, G. M. et al. Histologic analysis of the irradiated anal sphincter. Dis. Colon Rectum 46, 1492–1497 (2003).
Bregendahl, S. et al. Neorectal hyposensitivity after neoadjuvant therapy for rectal cancer. Radiother. Oncol. 108, 331–336 (2013).
Haas, S. et al. Anal sphincter dysfunction in patients treated with primary radiotherapy for anal cancer: a study with the functional lumen imaging probe. Acta Oncologica 57, 465–472 (2018).
Vordermark, D., Sailer, M., Flentje, M., Thiede, A. & Kölbl, O. Curative-intent radiation therapy in anal carcinoma: quality of life and sphincter function. Radiother. Oncol. 52, 239–243 (1999).
Dalsania, R. M., Shah, K. P., Stotsky-Himelfarb, E., Hoffe, S. & Willingham, F. F. Management of long-term toxicity from pelvic radiation therapy. Am. Soc. Clin. Oncol. Educ. Book 41, 1–11 (2021).
Smart, A. C. et al. Gender-affirming surgery and cancer: considerations for radiation oncologists for pelvic radiation in transfeminine patients. Int. J. Radiat. Oncol. Biol. Phys. 117, 301–311 (2023).
Antonelli, R. Q. et al. Liquid silicone used for esthetic purposes as a potentiator for occurrence of post-radiotherapy genital lymphedema: case report. Sao Paulo Med. J. 135, 185–189 (2017).
Dickstein, D. R. & Buckstein, M. What rectal cancer patients may be able to safely avoid radiation? Curr. Colorectal Cancer Rep. 18, 61–67 (2022).
Bertoncelli Tanaka, M. et al. Prostate cancer in transgender women: what does a urologist need to know? BJU Int. 129, 113–122 (2022).
Sinawe, H. & Casadesus, D. Mitomycin. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK562249/ (updated 24 Jul 2023).
Casale, J. & Patel, P. Fluorouracil. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK549808/ (updated 24 Nov 2023).
Marsé, H., Van Cutsem, E., Grothey, A. & Valverde, S. Management of adverse events and other practical considerations in patients receiving capecitabine (Xeloda). Eur. J. Oncol. Nurs. 8, S16–S30 (2004).
Garrison, S. R. et al. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 9, CD009402 (2020).
Haris, I. et al. Sexual dysfunction following breast cancer chemotherapy: a cross-sectional study in Yogyakarta, Indonesia. Cureus 15, e41744 (2023).
Grisold, W., Cavaletti, G. & Windebank, A. J. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-Oncology 14, iv45–iv54 (2012).
Zajączkowska, R. et al. Mechanisms of chemotherapy-induced peripheral neuropathy. Int. J. Mol. Sci. 20, 1451 (2019).
Voznesensky, M., Annam, K. & Kreder, K. J. Understanding and managing erectile dysfunction in patients treated for cancer. J. Oncol. Pract. 12, 297–304 (2016).
Boyette-Davis, J. A., Hou, S., Abdi, S. & Dougherty, P. M. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 8, 363–375 (2018).
Cercek, A. et al. PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Dhawan, N., Afzal, M. Z. & Amin, M. Immunotherapy in anal cancer. Curr. Oncol. 30, 4538–4550 (2023).
Grover, S., Wang, Y. & Dougan, M. Immune checkpont inhibitor colitis. UpToDate https://www.uptodate.com/contents/immune-checkpoint-inhibitor-colitis (2023).
Olusoji, R. A., Eun, Y., Mohamed Salih, R., Osei, N. & Ahluwalia, M. Adrenal insufficiency in patients on immune checkpoint inhibitors: an all of Us data study. J. Clin. Oncol. 41, e14675 (2023).
Basch, E. et al. Patient-reported outcomes during and after treatment for locally advanced rectal cancer in the PROSPECT trial (Alliance N1048). J. Clin. Oncol. 41, 3724–3734 (2023).
Garcia-Aguilar, J. et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J. Clin. Oncol. 40, 2546–2556 (2022).
Gerard, J.-P. et al. Neoadjuvant chemoradiotherapy with radiation dose escalation with contact x-ray brachytherapy boost or external beam radiotherapy boost for organ preservation in early cT2–cT3 rectal adenocarcinoma (OPERA): a phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 8, 356–367 (2023).
Schrag, D. et al. Preoperative treatment of locally advanced rectal cancer. N. Engl. J. Med. 389, 322–334 (2023).
Herbenick, D. et al. Women’s sexual satisfaction, communication, and reasons for (no longer) faking orgasm: findings from a U.S. probability sample. Arch. Sex. Behav. 48, 2461–2472 (2019).
Beaulieu, N., Bergeron, S., Brassard, A., Byers, E. S. & Péloquin, K. Toward an integrative model of intimacy, sexual satisfaction, and relationship satisfaction: a prospective study in long-term couples. J. Sex. Res. 60, 1100–1112 (2023).
Rosenberger, J. G., Schick, V., Herbenick, D., Novak, D. S. & Reece, M. Sex toy use by gay and bisexual men in the United States. Arch. Sex. Behav. 41, 449–458 (2012).
Swan, E. Colostomy, management and quality of life for the patient. Br. J. Nurs. 20, 24–28 (2011).
Godala, M., Gaszyńska, E., Durko, Ł. & Małecka-Wojciesko, E. Dietary behaviors and beliefs in patients with inflammatory bowel disease. J. Clin. Med. 12, 3455 (2023).
Karagama, Y. Abelchia: inability to belch/burp-a new disorder? Retrograde cricopharyngeal dysfunction (RCPD). Eur. Arch. Otorhinolaryngol. 278, 5087–5091 (2021).
Lin, S. Y. et al. Different position from traditional left lateral for colonoscopy? A meta-analysis and systematic review of randomized control trials. Chronic Dis. Transl. Med. 7, 27–34 (2021).
Ralph, S. Developing UK guidance on how long men should abstain from receiving anal sex before, during and after interventions for prostate cancer. Clin. Oncol. 33, 807–810 (2021).
Mlakar, B. Should we avoid stapled hemorrhoidopexy in males and females who practice receptive anal sex? Dis. Colon Rectum 50, 1727 (2007).
Carballo-Diéguez, A., Lentz, C., Giguere, R., Fuchs, E. J. & Hendrix, C. W. Rectal douching associated with receptive anal intercourse: a literature review. AIDS Behav. 22, 1288–1294 (2018).
Leyva, F. J. et al. Isoosmolar enemas demonstrate preferential gastrointestinal distribution, safety, and acceptability compared with hyperosmolar and hypoosmolar enemas as a potential delivery vehicle for rectal microbicides. AIDS Res. Hum. Retroviruses 29, 1487–1495 (2013).
Saleska, J. L. et al. Correlates of rectal douching practices among men who have sex with men in Kenya. Sex. Transm. Dis. 45, e94–e97 (2018).
Mayer, K. H. et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 396, 239–254 (2020).
Landovitz, R. J. et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N. Engl. J. Med. 385, 595–608 (2021).
Alrubia, S., Mao, J., Chen, Y., Barber, J. & Rostami-Hodjegan, A. Altered bioavailability and pharmacokinetics in Crohn’s disease: capturing systems parameters for PBPK to assist with predicting the fate of orally administered drugs. Clin. Pharmacokinet. 61, 1365–1392 (2022).
Effinger, A., O’Driscoll, C. M., McAllister, M. & Fotaki, N. Impact of gastrointestinal disease states on oral drug absorption — implications for formulation design — a PEARRL review. J. Pharm. Pharmacol. 71, 674–698 (2019).
Moss, D. M., Curley, P., Shone, A., Siccardi, M. & Owen, A. A multisystem investigation of raltegravir association with intestinal tissue: implications for pre-exposure prophylaxis and eradication. J. Antimicrob. Chemother. 69, 3275–3281 (2014).
Brooks, K. M. et al. Decreased absorption of dolutegravir and tenofovir disoproxil fumarate, but not emtricitabine, in an HIV-infected patient following oral and jejunostomy-tube administration. Pharmacotherapy 37, e82–e89 (2017).
Calcagno, A. et al. Low tenofovir plasma exposure in HIV oral pre-exposure prophylaxis recipients with gastrointestinal disorders. Antimicrob. Agents Chemother. 65, e01902-20 (2021).
Molina, J. M. et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect. Dis. 18, 308–317 (2018).
Kong, F. Y. S., Kenyon, C. & Unemo, M. Important considerations regarding the widespread use of doxycycline chemoprophylaxis against sexually transmitted infections. J. Antimicrob. Chemother. 78, 1561–1568 (2023).
Siegler, A. J. et al. Levels of clinical condom failure for anal sex: a randomized cross-over trial. EClinicalMedicine 17, 100199 (2019).
Fuchs, E. J. et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J. Infect. Dis. 195, 703–710 (2007).
Hardy, D. When We Dare Not Speak its Name: Anal Taboo, Anal Health, and Affect Theory (Widener University, 2010).
Dezzutti, C. S. et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS ONE 7, e48328 (2012).
White, S. Gay relationships and the challenges a stoma can cause. Securi Care https://www.securicaremedical.co.uk/blog/gay-relationships-and-the-challenges-a-stoma-can-cause (2020).
Lim, S. W. et al. Risk factors for permanent stoma after low anterior resection for rectal cancer. Langenbecks Arch. Surg. 398, 259–264 (2013).
Pellino, G., Sciaudone, G., Candilio, G., Canonico, S. & Selvaggi, F. Rectosigmoid stump washout as an alternative to permanent mucous fistula in patients undergoing subtotal colectomy for ulcerative colitis in emergency settings. BMC Surg. 12, S31 (2012).
NHS. Living with Colostomy. NHS https://www.nhs.uk/conditions/colostomy/living-with/ (2023).
Exploring Gay Intimacy with an Ostomy Bag: Is it Feasible? Meet An OstoMate https://www.meetanostomate.org/discussion-forum/viewtopic.php?t=10018 (2023).
Bhaskar, V., Sinha, R. J., Mehrotra, S., Mehrotra, C. N. & Singh, V. Long-term outcomes of sigmoid vaginoplasty in patients with disorder of sexual development — our experience. Urol. Ann. 10, 185–190 (2018).
Krakowsky, Y. et al. The effect of gender-affirming medical care on the vaginal and neovaginal microbiomes of transgender and gender-diverse people. Front. Cell Infect. Microbiol. 11, 769950 (2021).
Jones, E. S. & Rayner, B. L. The importance of guidelines. Cardiovasc. J. Afr. 25, 296–297 (2014).
Albrecht, H. & Gretschel, S. Laparoscopic sphincter reconstruction after abdominoperineal resection: feasibility and technical aspects. Tech. Coloproctol. 23, 367–372 (2019).
van Netten, J. J., Georgiadis, J. R., Nieuwenburg, A. & Kortekaas, R. 8–13 Hz fluctuations in rectal pressure are an objective marker of clitorally-induced orgasm in women. Arch. Sex. Behav. 37, 279–285 (2008).
Matzel, K. E. Update on neuromodulation for fecal incontinence. Ann. Laparosc. Endosc. Surg. https://doi.org/10.21037/ales-2022-03 (2022).
Brill, S. A. & Margolin, D. A. Sacral nerve stimulation for the treatment of fecal incontinence. Clin. Colon Rectal Surg. 18, 38–41 (2005).
Morin, M. et al. Multimodal physical therapy versus topical lidocaine for provoked vestibulodynia: a multicenter, randomized trial. Am. J. Obstet. Gynecol. 224, 189.e1–189.e12 (2021).
Keefer, L. Behavioural medicine and gastrointestinal disorders: the promise of positive psychology. Nat. Rev. Gastroenterol. Hepatol. 15, 378–386 (2018).
Rosen, N. O., Bergeron, S., Sadikaj, G. & Delisle, I. Daily associations among male partner responses, pain during intercourse, and anxiety in women with vulvodynia and their partners. J. Pain 16, 1312–1320 (2015).
Morais, L. H., Schreiber, H. L. & Mazmanian, S. K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255 (2021).
Keefer, L. et al. A Rome Working Team report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology 162, 300–315 (2022).
Meana, M. & Binik, Y. M. The biopsychosocial puzzle of painful sex. Annu. Rev. Clin. Psychol. 18, 471–495 (2022).
Paulton, J., Gill, A. & Prevost, J. Gut-directed self-hypnosis for inflammatory bowel disease protocol: complimentary psychotherapy for remission augmentation, IBS-like symptoms, and surgery recovery. Gastroenterology 160, S72 (2021).
Eberspacher, C. et al. Self-mechanical anal dilatation: a simple trick to minimize postoperative pain and stenosis following hemorrhoidectomy with radiofrequency. Front. Surg. 8, 711958 (2021).
Grimstad, F., McLaren, H. & Gray, M. The gynecologic examination of the transfeminine person after penile inversion vaginoplasty. Am. J. Obstet. Gynecol. 224, 266–273 (2021).
Bakker, R. M. et al. Sexual rehabilitation after pelvic radiotherapy and vaginal dilator use: consensus using the Delphi method. Int. J. Gynecol. Cancer 24, 1499–1506 (2014).
Summerfield, J. & Leong, A. Management of radiation therapy-induced vaginal adhesions and stenosis: a New Zealand survey of current practice. J. Med. Radiat. Sci. 67, 128–133 (2020).
Lee, Y. Patients’ perception and adherence to vaginal dilator therapy: a systematic review and synthesis employing symbolic interactionism. Patient Prefer. Adherence 12, 551–560 (2018).
Bianco, F. et al. Short stump and high anastomosis pull-through (SHiP) procedure for delayed coloanal anastomosis with no protective stoma for low rectal cancer. Updates Surg. 73, 495–502 (2021).
Greca, F., Nevah, E., Hares, M. & Keighley, M. R. Value of an anal dilator after anal stretch for haemorrhoids. J. R. Soc. Med. 74, 368–370 (1981).
Shen, S. L., He, D. L. & Luo, Y. Clinical trials of combined therapy of an oral Chinese medicine with massage for chronic nonbacterial prostatitis [Chinese]. Zhonghua Nan Ke Xue 12, 851–853 (2006).
Ateya, A. et al. Evaluation of prostatic massage in treatment of chronic prostatitis. Urology 67, 674–678 (2006).
Moskowitz, D. A. & Garcia, C. P. Top, bottom, and versatile anal sex roles in same-sex male relationships: implications for relationship and sexual satisfaction. Arch. Sex. Behav. 48, 1217–1225 (2019).
Wellings, K. & Johnson, A. M. Framing sexual health research: adopting a broader perspective. Lancet 382, 1759–1762 (2013).
Maister, L., Fotopoulou, A., Turnbull, O. & Tsakiris, M. The erogenous mirror: intersubjective and multisensory maps of sexual arousal in men and women. Arch. Sex. Behav. 49, 2919–2933 (2020).
Turnbull, O. H., Lovett, V. E., Chaldecott, J. & Lucas, M. D. Reports of intimate touch: erogenous zones and somatosensory cortical organization. Cortex 53, 146–154 (2014).
Polter, E. J. et al. Creation and psychometric validation of the sexual minorities and prostate cancer scale (SMACS) in sexual minority patients-the restore-2 study. J. Sex. Med. 19, 529–540 (2022).
Flynn, K. E., Lin, L. & Weinfurt, K. P. Sexual function and satisfaction among heterosexual and sexual minority U.S. adults: a cross-sectional survey. PLoS ONE 12, e0174981 (2017).
Weinfurt, K. P. et al. Development and initial validation of the PROMIS® sexual function and satisfaction measures version 2.0. J. Sex. Med. 12, 1961–1974 (2015).
Fatton, B., de Tayrac, R., Letouzey, V. & Huberlant, S. Pelvic organ prolapse and sexual function. Nat. Rev. Urol. 17, 373–390 (2020).
Gül, M. et al. A clinical guide to rare male sexual disorders. Nat. Rev. Urol. 21, 35–49 (2023).
Vriesman, M. H., Koppen, I. J. N., Camilleri, M., Di Lorenzo, C. & Benninga, M. A. Management of functional constipation in children and adults. Nat. Rev. Gastroenterol. Hepatol. 17, 21–39 (2020).
Rao, S. S. & Patcharatrakul, T. Diagnosis and treatment of dyssynergic defecation. J. Neurogastroenterol. Motil. 22, 423–435 (2016).
Drolet, M., Bénard, É., Pérez, N. & Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 394, 497–509 (2019).
Yakely, A. E., Avni-Singer, L., Oliveira, C. R. & Niccolai, L. M. Human papillomavirus vaccination and anogenital warts: a systematic review of impact and effectiveness in the United States. Sex. Transm. Dis. 46, 213–220 (2019).
Koff, R. S. Hepatitis A. Lancet 351, 1643–1649 (1998).
Spratt, D. E. et al. Vessel-sparing radiotherapy for localized prostate cancer to preserve erectile function: a single-arm phase 2 trial. Eur. Urol. 72, 617–624 (2017).
Pauls, R. N. Anatomy of the clitoris and the female sexual response. Clin. Anat. 28, 376–384 (2015).
Di Marino, V. & Lepidi, H. Anatomic Study of the Clitoris and the Bulbo-Clitoral Organ 1st edn (Springer International Publishing, 2014).
Jannini, E. A., Buisson, O. & Rubio-Casillas, A. Beyond the G-spot: clitourethrovaginal complex anatomy in female orgasm. Nat. Rev. Urol. 11, 531–538 (2014).
Kinter, K. J. & Newton, B. W. Abdomen and Pelvis, Pudendal Nerve. StatPearls [Internet] https://www.ncbi.nlm.nih.gov/books/NBK554736/ (updated 10 Feb 2023).
Maravilla, K. R. et al. Serial MR imaging with MS-325 for evaluating female sexual arousal response: determination of intrasubject reproducibility. J. Magn. Reson. Imaging 18, 216–224 (2003).
Shafik, A., Shafik, I. A., el-Sibai, O. & Shafik, A. A. Physioanatomical relationship of the external anal sphincter to the bulbocavernosus muscle in the female. Int. Urogynecol. J. Pelvic Floor Dysfunct. 18, 851–856 (2007).
Arias-Castillo, L., García, L. & García-Perdomo, H. A. The complexity of female orgasm and ejaculation. Arch. Gynecol. Obstet. 308, 427–434 (2023).
Alwaal, A., Breyer, B. N. & Lue, T. F. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil. Steril. 104, 1051–1060 (2015).
Djinovic, R. P. Metoidioplasty. Clin. Plast. Surg. 45, 381–386 (2018).
Djordjevic, M. L., Stojanovic, B. & Bizic, M. Metoidioplasty: techniques and outcomes. Transl. Androl. Urol. 8, 248–253 (2019).
Hage, J. J. & van Turnhout, A. A. Long-term outcome of metaidoioplasty in 70 female-to-male transsexuals. Ann. Plast. Surg. 57, 312–316 (2006).
Cristofari, S. et al. Genitourinary vascularized composite allotransplantation for gender affirmation in trans men: an anatomical feasibility study. J. Plast. Reconstr. Aesthet. Surg. 83, 117–125 (2023).
Marque, M. et al. The tube in tube thoracodorsal perforator flap phalloplasty [French]. Ann. Chir. Plast. Esthet. https://doi.org/10.1016/j.anplas.2023.01.003 (2023).
Monstrey, S. J., Ceulemans, P. & Hoebeke, P. Sex reassignment surgery in the female-to-male transsexual. Semin. Plast. Surg. 25, 229–244 (2011).
Elfering, L. et al. How sensitive is the neophallus? Postphalloplasty experienced and objective sensitivity in transmasculine persons. Sex. Med. 9, 100413 (2021).
Canale, D. et al. Genital sensitivity and perceived orgasmic intensity in transgender women with gender dysphoria after gender-affirming surgery: a pilot study comparing pelvic floor evoked somatosensory potentials and patient subjective experience. J. Sex. Med. 19, 1479–1487 (2022).
Kloer, C. et al. Sexual health after vaginoplasty: a systematic review. Andrology 9, 1744–1764 (2021).
CDC. Discussing Sexual Health with Your Patients. CDC https://www.cdc.gov/stophivtogether/library/topics/prevention/brochures/cdc-lsht-prevention-brochure-clinicians-quick-guide-discussing-sexual-health-your-patients.pdf (2022).
Hein, L. C., & Levitt, N. Caring for…transgender patients. Nurs. Made Incredibly Easy 12, 28–36 (2014).
Bauer, G. R., Redman, N., Bradley, K. & Scheim, A. I. Sexual health of trans men who are gay, bisexual, or who have sex with men: results from Ontario, Canada. Int. J. Transgend. 14, 66–74 (2013).
Kenagy, G. P. & Hsieh, C. M. The risk less known: female-to-male transgender persons’ vulnerability to HIV infection. AIDS Care 17, 195–207 (2005).
Rowniak, S., Chesla, C., Rose, C. D. & Holzemer, W. L. Transmen: the HIV risk of gay identity. AIDS Educ. Prev. 23, 508–520 (2011).
Sevelius, J. “There’s no pamphlet for the kind of sex I have”: HIV-related risk factors and protective behaviors among transgender men who have sex with nontransgender men. J. Assoc. Nurses AIDS Care 20, 398–410 (2009).
Nemoto, T., Bödeker, B., Iwamoto, M. & Sakata, M. Practices of receptive and insertive anal sex among transgender women in relation to partner types, sociocultural factors, and background variables. AIDS Care 26, 434–440 (2014).
Dangerfield, D. T. II, Smith, L. R., Williams, J., Unger, J. & Bluthenthal, R. Sexual positioning among men who have sex with men: a narrative review. Arch. Sex. Behav. 46, 869–884 (2017).
Ock, M., Lim, S. Y., Jo, M. W. & Lee, S. I. Frequency, expected effects, obstacles, and facilitators of disclosure of patient safety incidents: a systematic review. J. Prev. Med. Public Health 50, 68–82 (2017).
Weiss, P. M. & Miranda, F. Transparency, apology and disclosure of adverse outcomes. Obstet. Gynecol. Clin. North Am. 35, 53–62 (2008).
Basch, E., Barbera, L., Kerrigan, C. L. & Velikova, G. Implementation of patient-reported outcomes in routine medical care. Am. Soc. Clin. Oncol. Educ. Book 38, 122–134 (2018).
Basch, E. et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318, 197–198 (2017).
Basch, E. et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J. Clin. Oncol. 34, 557–565 (2016).
Oggesen, B. T., Hamberg, M. L. S., Thomsen, T. & Rosenberg, J. Exploring patients’ perspectives on late complications after colorectal and anal cancer treatment: a qualitative study. Curr. Oncol. 30, 7532–7541 (2023).
The National Academies Collection: Reports Funded by National Institutes of Health (eds Becker, T., Chin, M. & Bates, N.) (National Academies Press, 2022).
Orkin, B. A., Sinykin, S. B. & Lloyd, P. C. The digital rectal examination scoring system (DRESS). Dis. Colon Rectum 53, 1656–1660 (2010).
Serati, M., Espuña-Pons, M., Mouton-Puglisi, A. & Padoa, A. Iron deficiency and sexual dysfunction in women. Sex. Med. Rev. 27, 342–348 (2023).
Gulmez, H. et al. Impact of iron supplementation on sexual dysfunction of women with iron deficiency anemia in short term: a preliminary study. J. Sex. Med. 11, 1042–1046 (2014).
Wiegel, M., Meston, C. & Rosen, R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J. Sex Marital Ther. 31, 1–20 (2005).
Tirabassi, G. et al. Vitamin D and male sexual function: a transversal and longitudinal study. Int. J. Endocrinol. 2018, 3720813 (2018).
Krysiak, R., Szwajkosz, A., Marek, B. & Okopień, B. The effect of vitamin D supplementation on sexual functioning and depressive symptoms in young women with low vitamin D status. Endokrynol. Pol. 69, 168–174 (2018).
Carrington, E. V. et al. Advances in the evaluation of anorectal function. Nat. Rev. Gastroenterol. Hepatol. 15, 309–323 (2018).
Bardin, M. G. et al. Clitoral blood flow using color Doppler ultrasonography in women with and without provoked vestibulodynia. Int. Urogynecol. J. 33, 1489–1494 (2022).
Heitmann, P. T. et al. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat. Rev. Gastroenterol. Hepatol. 18, 751–769 (2021).
Wang, X. T. et al. Meta-analysis of oncological outcome after abdominoperineal resection or low anterior resection for lower rectal cancer. Pathol. Oncol. Res. 21, 19–27 (2015).
Moszkowicz, D. et al. Where does pelvic nerve injury occur during rectal surgery for cancer? Colorectal Dis. 13, 1326–1334 (2011).
George, D., Pramil, K., Kamalesh, N. P., Ponnambatheyil, S. & Kurumboor, P. Sexual and urinary dysfunction following laparoscopic total mesorectal excision in male patients: a prospective study. J. Minim. Access. Surg. 14, 111–117 (2018).
Bae, S. U., Varela, C., Nassr, M. & Kim, N. K. Customized Denonvilliers’ fascia excision: an advanced total mesorectal excision technique for anteriorly located rectal cancer. Dis. Colon Rectum 66, e304–e309 (2023).
Yun, S. J. et al. Vitiligo-like depigmentation after pembrolizumab treatment in patients with non-small cell lung cancer: a case report. Transl. Lung Cancer Res. 9, 1585–1590 (2020).
Acknowledgements
The authors thank A. Fenner (Nature Reviews Urology) for providing initial support. D.C.M. is supported by the Office of The Director, National Institutes of Health, under Award Number DP5OD031876. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made a substantial contribution to the discussion of content, and reviewed and edited the article before submission. D.R.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.E.G. is an investigator on the human papillomavirus vaccine and consultant for Merck. C.R.R. reports speaker training with Takeda. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Marco Romano, Laura Targownik and Christopher Velez for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Anodyspareunia
-
Painful receptive anal intercourse.
- Anorgasmia
-
Failure to achieve orgasm or the experience of weakened or diminished orgasms.
- Bottom
-
The receptive partner in anal intercourse; although this term has also been generalized in sexual minority culture to include the receptive partner in oral intercourse.
- Bottom-shaming
-
Judging, demeaning or devaluing someone for deriving pleasure from receptive anal intercourse.
- Bulboclitoris
-
Clitoris and vestibular bulbs.
- Butthurt
-
A colloquialism used to describe someone who is overly sensitive, potentially originating from a sensitive child who is spanked, but now also connotating that people who engage in receptive anal intercourse (RAI) might be sensitive and that RAI is painful, thus minimizing the association of pleasure and RAI.
- Dysorgasmia
-
Pain during orgasm.
- Neoanus
-
A reconstructed or created anus.
- Neoclitoris
-
A reconstructed or created clitoris.
- Neophallus
-
A reconstructed or created phallic structure, typically through metoidioplasty or phalloplasty.
- Neovagina
-
A reconstructed or created vagina.
- Pegging
-
Pleasurable receptive anal intercourse experienced through stimulation by an attachable artificial phallic object on a partner; commonly but not exclusively used in the context of a cisgender heterosexual male receiving anal pleasure from a cisgender woman with a strap-on dildo.
- Rimming
-
Pleasurable receptive anal intercourse experienced through stimulation by the tongue or mouth of a partner.
- Role-in-sex
-
The role a person identifies with during sexual intercourse (for example, top, bottom, versatile, side).
- Sexual and gender minority
-
Individuals who identify as lesbian, gay, bisexual, transgender, gender diverse, asexual, queer or intersex as well as those who do not but whose sexual orientation, gender identity or reproductive development varies from traditional, societal, cultural or physiological norms.
- Shower shot
-
Anorectal douching using an enema attached to a shower.
- Side
-
A person who does not identify with “top”, “bottom”, or “vers” and might not engage in anal intercourse.
- Social determinants of health
-
The environmental conditions in which people are born, reside, learn, work, worship, engage in recreational activities and age that influence health outcomes.
- Top
-
The insertive partner in anal intercourse; although this term has been generalized in sexual minority culture to also include the insertive partner in oral intercourse.
- Vers
-
Or verse, short for ‘versatile’, a person who engages in both the receptive and insertive role in intercourse.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dickstein, D.R., Edwards, C.R., Rowan, C.R. et al. Pleasurable and problematic receptive anal intercourse and diseases of the colon, rectum and anus. Nat Rev Gastroenterol Hepatol 21, 377–405 (2024). https://doi.org/10.1038/s41575-024-00932-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-024-00932-1