Matters Arising
|
Open Access
Featured
-
-
Article
| Open Access Discovery and biosynthesis of tricyclic copper-binding ribosomal peptides containing histidine-to-butyrine crosslinks
Discovery and biosynthesis of tricyclic copper-binding ribosomal peptides containing histidine-to-butyrine crosslinks
Cyclic peptides are important bioactive compounds and drugs, synthesised by enzymatic side-chain macrocyclization of ribosomal peptides, which rarely involves histidine residues. Here, the authors report the discovery and biosynthesis of tricyclic lanthipeptide noursin, constrained by a tri amino acid labionin crosslink and histidine-to-butyrine crosslink, which is important for copper binding of noursin.
- Yuqing Li
- , Yeying Ma
- & Huan Wang
-
Article
| Open Access Ant venoms contain vertebrate-selective pain-causing sodium channel toxins
Ant venoms contain vertebrate-selective pain-causing sodium channel toxins
Stings of certain ant species can cause intense, long-lasting nociception. Here, authors show that the major contributors of these symptoms are vertebrate-selective defensive venom peptides which modulate the activity of voltage-gated sodium channels.
- Samuel D. Robinson
- , Jennifer R. Deuis
- & Irina Vetter
-
Article
| Open Access Computational design of dynamic receptor—peptide signaling complexes applied to chemotaxis
Computational design of dynamic receptor—peptide signaling complexes applied to chemotaxis
Engineering protein biosensors that respond to biomolecules by triggering cellular responses has largely relied on binding rigid molecules. Here, the authors develop a computational strategy for designing signaling complexes between conformationally dynamic proteins and peptides.
- Robert E. Jefferson
- , Aurélien Oggier
- & Patrick Barth
-
Article
| Open Access High-affinity peptides developed against calprotectin and their application as synthetic ligands in diagnostic assays
High-affinity peptides developed against calprotectin and their application as synthetic ligands in diagnostic assays
A peptide was developed that binds to calprotectin, a marker of major inflammatory disorders, and found to be suited for diagnostic tests. The use of synthetic peptides in assays is of great interest due to their high precision, robustness and low price.
- Cristina Díaz-Perlas
- , Benjamin Ricken
- & Christian Heinis
-
Article
| Open Access Large-scale phage-based screening reveals extensive pan-viral mimicry of host short linear motifs
Large-scale phage-based screening reveals extensive pan-viral mimicry of host short linear motifs
Protein-protein interactions underlie all aspects of a viral infection. Here the authors employ a pan-viral approach for systematic identification of motif-mediated interactions between viral and human proteins and show that the information can be used to find targets for antiviral drug development.
- Filip Mihalič
- , Leandro Simonetti
- & Ylva Ivarsson
-
Article
| Open Access Low complexity domains of the nucleocapsid protein of SARS-CoV-2 form amyloid fibrils
Low complexity domains of the nucleocapsid protein of SARS-CoV-2 form amyloid fibrils
Here, the authors show the amyloid formation of a low-complexity domain of NCAP, and its potential for guiding the design of antiviral agents.
- Einav Tayeb-Fligelman
- , Jeannette T. Bowler
- & David S. Eisenberg
-
Article
| Open Access High-resolution separation of bioisomers using ion cloud profiling
High-resolution separation of bioisomers using ion cloud profiling
Ion mobility is used in mass spectrometers for structure analysis of biomolecules. Here, the authors show that ion mobility analysis in an ion trap under ultra-high fields enables isomer separation at resolutions over 10,000, wich they demonstrate for isomers of disaccharides, phospholipids, and peptides.
- Xiaoyu Zhou
- , Zhuofan Wang
- & Zheng Ouyang
-
Article
| Open Access Amide-to-ester substitution as a stable alternative to N-methylation for increasing membrane permeability in cyclic peptides
Amide-to-ester substitution as a stable alternative to N-methylation for increasing membrane permeability in cyclic peptides
Naturally occurring peptides with high membrane permeability often have backbone ester bonds. Here, the authors investigated the effect of an amide-to-ester substitution on membrane permeability of peptides and found the substitution is useful for improving membrane permeability of cyclic peptides.
- Yuki Hosono
- , Satoshi Uchida
- & Shinsuke Sando
-
Article
| Open Access Crocodile defensin (CpoBD13) antifungal activity via pH-dependent phospholipid targeting and membrane disruption
Crocodile defensin (CpoBD13) antifungal activity via pH-dependent phospholipid targeting and membrane disruption
Defensins are a class of host defence peptides that contribute to the immune system of eukaryotes. Here, the authors report the structure of saltwater crocodile defensin CpoBD13 and the mechanism of pH-dependent antifungal activity.
- Scott A. Williams
- , Fung T. Lay
- & Mark D. Hulett
-
Article
| Open Access A bivalent remipede toxin promotes calcium release via ryanodine receptor activation
A bivalent remipede toxin promotes calcium release via ryanodine receptor activation
Insect toxins with tandem repeats of neurotoxin domains have been found with enhanced receptor avidity. Here, the authors describe a bivalent toxin from remipede venom that targets ryanodine receptors, a rare target for animal venoms.
- Michael J. Maxwell
- , Chris Thekkedam
- & Mehdi Mobli
-
Article
| Open Access Biomolecular condensates formed by designer minimalistic peptides
Biomolecular condensates formed by designer minimalistic peptides
The molecular mechanisms underlying the formation of biomolecular condensates have not been fully elucidated. Here the authors show that the LLPS propensity, dynamics, and encapsulation efficiency of designed peptide condensates can be tuned by subtle changes to the peptide composition.
- Avigail Baruch Leshem
- , Sian Sloan-Dennison
- & Ayala Lampel
-
Article
| Open Access Intracellular phase separation of globular proteins facilitated by short cationic peptides
Intracellular phase separation of globular proteins facilitated by short cationic peptides
Phase separation provides intracellular organisation via membraneless entities called biomolecular condensates. Here, the authors show that short, cationic peptide tags can drive biomolecular condensation of engineered proteins in E. coli through associative interactions with RNA.
- Vivian Yeong
- , Jou-wen Wang
- & Allie C. Obermeyer
-
Article
| Open Access Thiocarbazate building blocks enable the construction of azapeptides for rapid development of therapeutic candidates
Thiocarbazate building blocks enable the construction of azapeptides for rapid development of therapeutic candidates
The rapid protease degradation of peptides is currently limiting their therapeutic utility. Here, the authors report functionalised thiocarbazate scaffolds as precursors of aza-amino acids that can be integrated in peptide sequences, extending their bioavailability, and demonstrate this on FSSE/P5779 and bradykinin.
- Ahmad Altiti
- , Mingzhu He
- & Yousef Al-Abed
-
Article
| Open Access AlphaPeptDeep: a modular deep learning framework to predict peptide properties for proteomics
AlphaPeptDeep: a modular deep learning framework to predict peptide properties for proteomics
Deep learning (DL) has been frequently used in mass spectrometry-based proteomics but there is still a lot of potential. Here, the authors develop a framework that enables building DL models to predict arbitrary peptide properties with only a few lines of code.
- Wen-Feng Zeng
- , Xie-Xuan Zhou
- & Matthias Mann
-
Article
| Open Access A glutamine-based single α-helix scaffold to target globular proteins
A glutamine-based single α-helix scaffold to target globular proteins
Targeting biomedically relevant protein-protein interactions is a long-lasting challenge in medicinal chemistry. Here, the authors develop a single α-helical peptide scaffold that can be tailored to target globular proteins of biomedical interest.
- Albert Escobedo
- , Jonathan Piccirillo
- & Xavier Salvatella
-
Article
| Open Access Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine
Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine
The modification of peptides and proteins for application in drug discovery and chemical biology is currently a rapidly growing field of research. Here, the authors report a photocatalytic diselenide contraction method for the dimerization and site-specific functionalisation of peptides and protein.
- Luke J. Dowman
- , Sameer S. Kulkarni
- & Richard J. Payne
-
Article
| Open Access A biomimetic electrostatic assistance for guiding and promoting N-terminal protein chemical modification
A biomimetic electrostatic assistance for guiding and promoting N-terminal protein chemical modification
Phosphorylation is a mechanism used by cells to promote proteins-biomolecules association. Here, the authors show the effect of the interactions between proteins equipped with positively charged arginines and peptides harbouring negatively charged phosphoserines, enabling rate acceleration and chemical processes in dilute conditions.
- Nathalie Ollivier
- , Magalie Sénéchal
- & Oleg Melnyk
-
Article
| Open Access Interaction of a viral insulin-like peptide with the IGF-1 receptor produces a natural antagonist
Interaction of a viral insulin-like peptide with the IGF-1 receptor produces a natural antagonist
The authors previously identified a family of viral insulin-like peptides (VILPs) with high homology to human insulin/IGF−1. Here, they report that one of these VILPs exhibits antagonist properties associated with a unique conformation of the IGF1R.
- Francois Moreau
- , Nicholas S. Kirk
- & C. Ronald Kahn
-
Article
| Open Access Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis
Unexpected assembly machinery for 4(3H)-quinazolinone scaffold synthesis
4(3H)-quinazolinone is the core scaffold in more than 200 natural alkaloids and numerous drugs. Here, the authors show an alternative assembly machinery for 4(3H)-quinazolinone mainly includes a two-module NRPS catalysing tripeptide formation, unusual N-3 original sources and an α-KGD catalysing the C-N bond oxidative cleavage of a tripeptide to form a dipeptide.
- Xi-Wei Chen
- , Li Rao
- & Yi Zou
-
Article
| Open Access Translation and natural selection of micropeptides from long non-canonical RNAs
Translation and natural selection of micropeptides from long non-canonical RNAs
Translation of 100 to 300 micropeptides from small ORFs within lncRNA was detected by Ribosomal Profiling in Drosophila embryos. These translated small ORFs showed natural selection conserving micropeptide sequence and function.
- Pedro Patraquim
- , Emile G. Magny
- & Juan Pablo Couso
-
Article
| Open Access The occurrence of ansamers in the synthesis of cyclic peptides
The occurrence of ansamers in the synthesis of cyclic peptides
The occurrence of isomers of the bicyclic octapeptide α-amanitin, which presents a macrolactam with a tryptathionine cross-link forming a handle, has been reported under the term of atropoisomers. Here, the authors synthesize its analogs and analyse their isomers, proposing and describing for them the term ansamer.
- Guiyang Yao
- , Simone Kosol
- & Roderich D. Süssmuth
-
Article
| Open Access Combining mass spectrometry and machine learning to discover bioactive peptides
Combining mass spectrometry and machine learning to discover bioactive peptides
Bioactive peptides regulate many physiological functions but progress in discovering them has been slow. Here, the authors use a machine learning framework to predict mammalian peptide candidates from the global and local structure of large-scale tissue-specific mass spectrometry data.
- Christian T. Madsen
- , Jan C. Refsgaard
- & Ulrik de Lichtenberg
-
Article
| Open Access Selective macrocyclic peptide modulators of Lys63-linked ubiquitin chains disrupt DNA damage repair
Selective macrocyclic peptide modulators of Lys63-linked ubiquitin chains disrupt DNA damage repair
Finding a selective modulator of Lys63-linked ubiquitin chains has proven very challenging. Here, the authors develop potent macrocyclic peptide binders of Lys63-linked di-ubiquitin chains that interrupt DNA damage repair and lead to apoptotic cell death.
- Ganga B. Vamisetti
- , Abhishek Saha
- & Ashraf Brik
-
Article
| Open Access A scalable platform to discover antimicrobials of ribosomal origin
A scalable platform to discover antimicrobials of ribosomal origin
Ribosomally synthesized and post-translationally modified peptides are a source of antimicrobials. Here, the authors report a platform for the rapid evaluation and characterization of biosynthetic gene clusters that enables the identification of 30 structurally diverse modified peptides, including three showing antimicrobial activities.
- Richard S. Ayikpoe
- , Chengyou Shi
- & Huimin Zhao
-
Article
| Open Access Activation of the insulin receptor by an insulin mimetic peptide
Activation of the insulin receptor by an insulin mimetic peptide
Genetic mutations of insulin receptor (IR) cause severe insulin resistance syndromes with no current treatment or cure. Here, the authors present that insulin-independent IR activation mechanism by peptide agonist which activate non-functional IR mutants that cause the insulin resistance syndromes.
- Junhee Park
- , Jie Li
- & Eunhee Choi
-
Article
| Open Access Insight into mode-of-action and structural determinants of the compstatin family of clinical complement inhibitors
Insight into mode-of-action and structural determinants of the compstatin family of clinical complement inhibitors
Therapeutic modulation of the complement system has gained interest over the past two decades. Here, the authors provide molecular-level insight into the mode-of-action, target selectivity and species specificity of the compstatin family of complement inhibitors, which entered the clinic in 2021.
- Christina Lamers
- , Xiaoguang Xue
- & Daniel Ricklin
-
Article
| Open Access Quantitative fragmentomics allow affinity mapping of interactomes
Quantitative fragmentomics allow affinity mapping of interactomes
Protein networks have been widely explored but most binding affinities remain unknown, limiting the quantitative interpretation of interactomes. Here the authors measure affinities of 65,000 interactions involving human PDZ domains and target sequence motifs relevant for viral infection and cancer.
- Gergo Gogl
- , Boglarka Zambo
- & Gilles Travé
-
Article
| Open Access Structural mechanism of tapasin-mediated MHC-I peptide loading in antigen presentation
Structural mechanism of tapasin-mediated MHC-I peptide loading in antigen presentation
The catalytic chaperone tapasin assists peptide loading onto MHC-I molecules for antigen presentation and immune recognition. Here, the authors present crystal structures that provide insights into the molecular mechanism of tapasin-mediated peptide exchange.
- Jiansheng Jiang
- , Daniel K. Taylor
- & Kannan Natarajan
-
Article
| Open Access Assembly of transmembrane pores from mirror-image peptides
Assembly of transmembrane pores from mirror-image peptides
Alpha-helix nanopores have a range of potential applications and the inclusion of non-natural amino acids allows for modification. Here, the authors report on the creation of alpha-helix pores using D-amino acids and show the pores formed, have different properties to the L-counterparts and were resistant to proteases.
- Smrithi Krishnan R
- , Kalyanashis Jana
- & Kozhinjampara R. Mahendran
-
Article
| Open Access A ribosomally synthesised and post-translationally modified peptide containing a β-enamino acid and a macrocyclic motif
A ribosomally synthesised and post-translationally modified peptide containing a β-enamino acid and a macrocyclic motif
The chemical diversity of peptides from ribosomal origin is a growing field of research. Here, the authors report the discovery, genomic and biosynthetic investigations of kintamdin, a ribosomally synthesized and post-translationally modified peptides featuring a beta-enamino acid and a bis-thioether macrocyclic motif.
- Shan Wang
- , Sixing Lin
- & Hai Deng
-
Article
| Open Access Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly
Designed peptides as nanomolar cross-amyloid inhibitors acting via supramolecular nanofiber co-assembly
Amyloid self-assembly is linked to type 2 diabetes and Alzheimer’s disease. Here the authors designed constrained peptides which are nanomolar amyloid inhibitors of the key polypeptides IAPP and Aβ42 and act via supramolecular nanofiber co-assembly.
- Karin Taş
- , Beatrice Dalla Volta
- & Aphrodite Kapurniotu
-
Article
| Open Access Control cell migration by engineering integrin ligand assembly
Control cell migration by engineering integrin ligand assembly
Engineering peptide assembly that controls integrin ligand presentation on the molecular level possesses by far the highest ligand density, expanding the perspective of ligand-density-dependent modulation.
- Xunwu Hu
- , Sona Rani Roy
- & Ye Zhang
-
Article
| Open Access Rational design of a sensitivity-enhanced tracer for discovering efficient APC–Asef inhibitors
Rational design of a sensitivity-enhanced tracer for discovering efficient APC–Asef inhibitors
The adenomatous polyposis coli (APC)–Asef protein interaction is essential for colorectal cancer metastasis. Here, the authors present the rational design of a sensitivity-enhanced tracer for fluorescence polarization assays, enabling them to discover more efficient APC–Asef interaction inhibitors.
- Jie Zhong
- , Yuegui Guo
- & Jian Zhang
-
Article
| Open Access Multifunctional synthetic nano-chaperone for peptide folding and intracellular delivery
Multifunctional synthetic nano-chaperone for peptide folding and intracellular delivery
Molecular chaperones play an important part in protein folding and delivery in nature. Here, the authors report on the creation of a synthetic chaperone to control the folding of therapeutic peptides from random coil to alpha helix and demonstrate enhanced therapeutic potential in an in vivo tumour model.
- Il-Soo Park
- , Seongchan Kim
- & Dal-Hee Min
-
Article
| Open Access Neprilysins regulate muscle contraction and heart function via cleavage of SERCA-inhibitory micropeptides
Neprilysins regulate muscle contraction and heart function via cleavage of SERCA-inhibitory micropeptides
Muscle contraction depends on strictly controlled calcium transients within myocytes. Here, the authors show that the endopeptidase Neprilysin 4 represents an essential regulator of these transients and, consequently, of proper heart function.
- Ronja Schiemann
- , Annika Buhr
- & Heiko Meyer
-
Article
| Open Access Mimicked synthetic ribosomal protein complex for benchmarking crosslinking mass spectrometry workflows
Mimicked synthetic ribosomal protein complex for benchmarking crosslinking mass spectrometry workflows
Cross-linking mass spectrometry is widely used to elucidate protein structures and interactions. Here, the authors generate an extensive peptide library to benchmark the most common cross-link search engines with frequently used cross-linking reagents in low and high complex sample systems.
- Manuel Matzinger
- , Adrian Vasiu
- & Karl Mechtler
-
Article
| Open Access A cryptic third active site in cyanophycin synthetase creates primers for polymerization
A cryptic third active site in cyanophycin synthetase creates primers for polymerization
Cyanophycin synthetase CphA1 polymerizes Asp and Arg into the nitrogen reserve polymer cyanophycin using two active sites. Sharon et al. show CphA1 has a cryptic 3rd active site that cleaves cyanophycin into primers for self-sufficient biosynthesis.
- Itai Sharon
- , Sharon Pinus
- & T. Martin Schmeing
-
Article
| Open Access Synthesis and direct assay of large macrocycle diversities by combinatorial late-stage modification at picomole scale
Synthesis and direct assay of large macrocycle diversities by combinatorial late-stage modification at picomole scale
Macrocycles have potential as therapeutics, but their libraries are currently not large enough for high-throughput screening. Here, the authors show a combinatorial approach to generate a library of almost 20’000 macrocycles by conjugating carboxylic-acid fragments to macrocyclic scaffolds, identifying nanomolar inhibitors against thrombin and binders of MDM2.
- Sevan Habeshian
- , Manuel Leonardo Merz
- & Christian Heinis
-
Article
| Open Access Hydrophobic-cationic peptides modulate RNA polymerase ribozyme activity by accretion
Hydrophobic-cationic peptides modulate RNA polymerase ribozyme activity by accretion
Macromolecular aggregates may have played an important role in the origin of life. Here, the authors report hydrophobic-cationic peptides that form insoluble aggregates, which reversibly accrete RNA on their surfaces, and enhance RNA polymerization by a ribozyme.
- Peiying Li
- , Philipp Holliger
- & Shunsuke Tagami
-
Article
| Open Access Ribosome inhibition by C9ORF72-ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM
Ribosome inhibition by C9ORF72-ALS/FTD-associated poly-PR and poly-GR proteins revealed by cryo-EM
The expansion of GGGGCC repeats in the C9ORF72 gene results in the production of disease causing abnormal proteins with polymeric glycine-arginine (poly-GR) and polymeric proline-arginine (poly-PR). Here the authors demonstrate a structural mechanism of how poly-GR and poly-PR inhibit translation and how they might also perturb ribosome assembly.
- Anna B. Loveland
- , Egor Svidritskiy
- & Andrei A. Korostelev
-
Article
| Open Access Thioesters provide a plausible prebiotic path to proto-peptides
Thioesters provide a plausible prebiotic path to proto-peptides
One of the early processes enabling the origins of life is thought to be the condensation of building blocks into oligomers and polymers. In this article, the authors report the synthesis of thiodepsipeptides and HS-peptides under mild temperatures and various pH, suggesting they could have formed on early prebiotic Earth.
- Moran Frenkel-Pinter
- , Marcos Bouza
- & Aikomari Guzman-Martinez
-
Article
| Open Access Molecular basis of antibiotic self-resistance in a bee larvae pathogen
Molecular basis of antibiotic self-resistance in a bee larvae pathogen
The authors show that the N-acetyltransferase PamZ acts as a self-resistance factor disabling the antibacterial paenilamicin that is produced by the honey bee larvae pathogen Paenibacillus larvae.
- Tam Dang
- , Bernhard Loll
- & Roderich D. Süssmuth
-
Article
| Open Access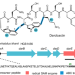 Radical SAM-dependent ether crosslink in daropeptide biosynthesis
Radical SAM-dependent ether crosslink in daropeptide biosynthesis
Darobactin is a ribosomally synthesized and post-translationally modified peptide featuring a unique scaffold and potent activity against Gram-negative bacteria. Here, the authors identify darobactin synthase DarE as responsible for the bicyclic scaffold formation and propose the name daropeptide for this growing class of enzymes.
- Sijia Guo
- , Shu Wang
- & Qi Zhang
-
Article
| Open Access De novo design and directed folding of disulfide-bridged peptide heterodimers
De novo design and directed folding of disulfide-bridged peptide heterodimers
Peptide heterodimers are prevalent in nature, which are not only functional macromolecules but molecular tools for chemical and synthetic biology. Here the authors report de novo design and directed folding of peptide heterodimers crosslinked through multiple disulfide bonds, which can be explored as chemical tools for orthogonal labeling of proteins and preparing protein hybrids.
- Sicong Yao
- , Adam Moyer
- & Chuanliu Wu
-
Article
| Open Access Molecular basis for allosteric agonism and G protein subtype selectivity of galanin receptors
Molecular basis for allosteric agonism and G protein subtype selectivity of galanin receptors
The basis for the diverse peptide-binding modes and the G protein selectivity of peptide GPCRs remains elusive. Here, the authors offer a structural basis for allosteric-like agonism and G protein selectivity of a neuropeptide GPCR, galanin receptor.
- Jia Duan
- , Dan-Dan Shen
- & Yi Jiang
-
Article
| Open Access Genotyping-by-sequencing-based identification of Arabidopsis pattern recognition receptor RLP32 recognizing proteobacterial translation initiation factor IF1
Genotyping-by-sequencing-based identification of Arabidopsis pattern recognition receptor RLP32 recognizing proteobacterial translation initiation factor IF1
Pattern-triggered immunity is activated by recognition of microbe-derived structures by host pattern recognition receptors. Here the authors use a genotype-by sequencing approach to show that bacterial translation initiation factor 1 triggers PTI in Arabidopsis thaliana after recognition by the RLP32 receptor.
- Li Fan
- , Katja Fröhlich
- & Thorsten Nürnberger
-
Article
| Open Access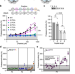 The YΦ motif defines the structure-activity relationships of human 20S proteasome activators
The YΦ motif defines the structure-activity relationships of human 20S proteasome activators
The proteasome complexes, composed of 20S core particles and one or two regulatory particles (proteasome activators), degrade most eukaryotic proteins. Here, the authors identify a sequence motif and resolve its interactions mediating the activation of the human 20S proteasome.
- Kwadwo A. Opoku-Nsiah
- , Andres H. de la Pena
- & Jason E. Gestwicki
-
Article
| Open Access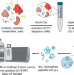 An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease
An efficient urine peptidomics workflow identifies chemically defined dietary gluten peptides from patients with celiac disease
Gluten peptides from wheat enter the bloodstream and are excreted in urine but are yet to be chemically characterised. Here, the authors show by mass spectrometry that quantitative and qualitative differences in urinary peptides can be detected between healthy people and patients with celiac disease.
- Brad A. Palanski
- , Nielson Weng
- & Joshua E. Elias
-
Article
| Open Access Unprotected peptide macrocyclization and stapling via a fluorine-thiol displacement reaction
Unprotected peptide macrocyclization and stapling via a fluorine-thiol displacement reaction
Strategies capable of stapling unprotected peptides in a straightforward, chemoselective, and clean manner, as well as promoting cellular uptake are of great interest. Here the authors report a peptide macrocyclization and stapling strategy which satisfies those criteria, based on a fluorine thiol displacement reaction.
- Md Shafiqul Islam
- , Samuel L. Junod
- & Rongsheng E. Wang
