« Prev Next »
Why 'nano'?
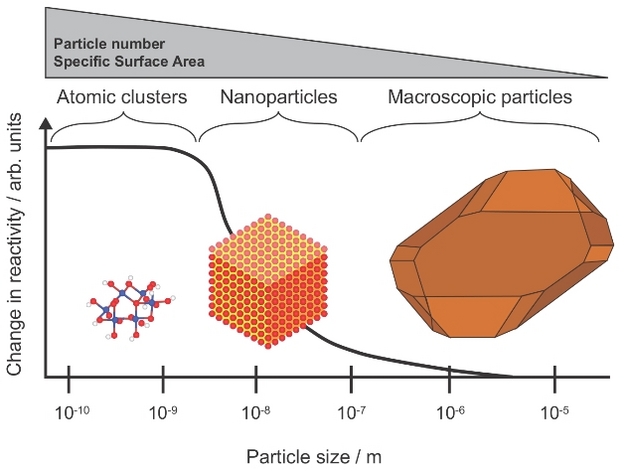
The term nanoparticle describes a subset of the colloidal range between 1 and 100 nm (Hochella 2002) (Box 1). The distinction is justified partly on their very high specific surface area (Lead & Wilkinson 2006) and partly on their potentially different behavior at this small scale, due to the spatial constraint of electronic properties (in an analogous manner to engineered nanoparticles (Madden et al. 2006)) (Figure 1). As particles transition to smaller and smaller sizes, they become effectively all surface with minimal internal volume, giving rise to their enhanced reactivity (Figure 1).

The environmental significance of colloids and nanoparticles resides in their interactions with trace metals (TM), through which they regulate the circulation and bioavailability of TMs in natural waters.
Nanoparticle and colloid types and characteristics
The most-studied subset of environmental organic matter, the humic substances (HS), are an operationally defined, chemically extracted fraction of the total natural organic carbon pool. Although not completely representative of the natural organic matter (NOM) (Filella 2008), in terms of trace metal binding, extracted HS behave similarly, although not identically, to their natural counterparts and may represent the most functionally significant component of NOM (Tipping 2002).
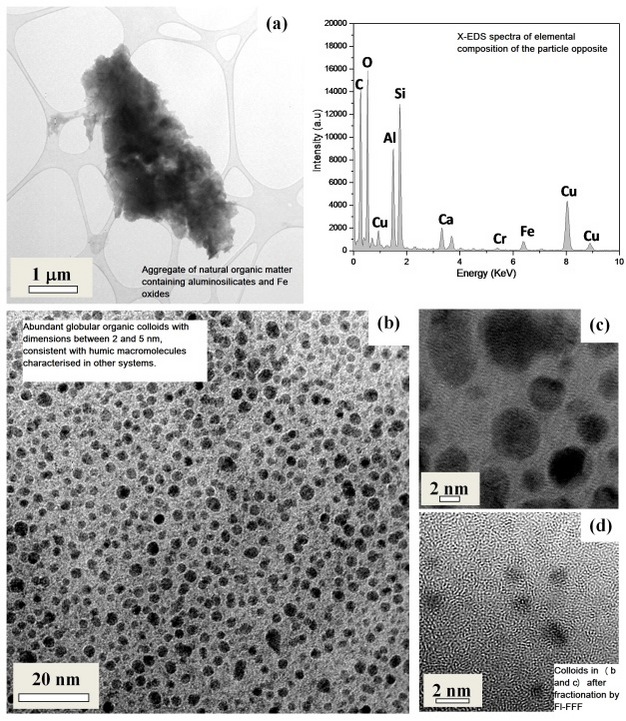
HS exist both as dispersed material at the lower end of the nano size range (< 5 nm) but can aggregate to form larger structures (often as composites with mineral colloids (e.g., Fe and Mn oxides and aluminosilicates), potentially reaching dimensions outside the nano-range (Stolpe & Hassellöv 2007) (Figure 2).
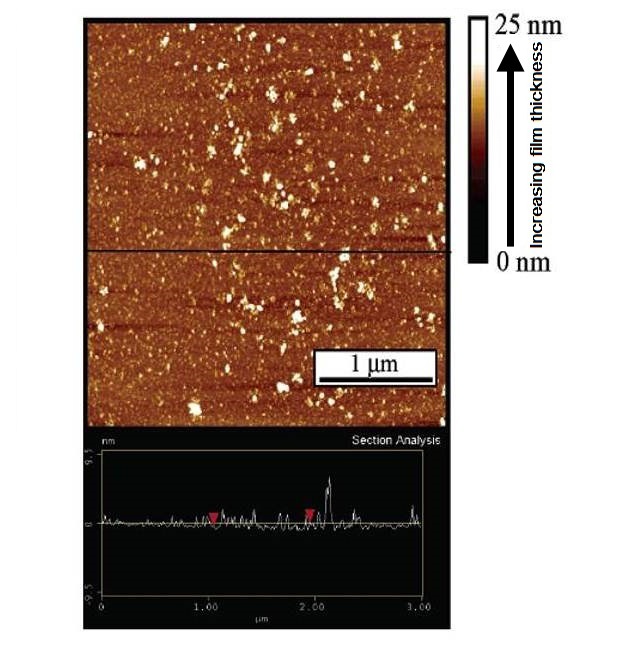
The next most abundant organic material found in natural waters consists of the peptides, proteins, peptidoglycans, polysaccharides and similar biomolecules. Polysaccharides have fibrillar or mesh-like configurations, whereas proteins are often globular and differ from HS in that they are ‘fresher,'-that is, less degraded. These types of compounds have the effect of generally increasing the size of nanoparticles and colloids through aggregation (Figure 4), while HS often, but not always, reduce aggregation through charge and/or steric stabilization (Buffle & Leppard 1995). In all environmental systems, metal oxides, especially of iron and manganese, are also important nano-scale phases, if not always in mass terms, then in their ability to bind trace elements (Figure 5).
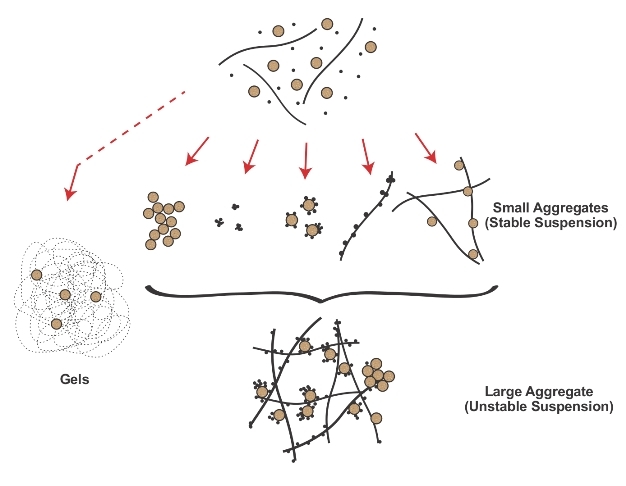
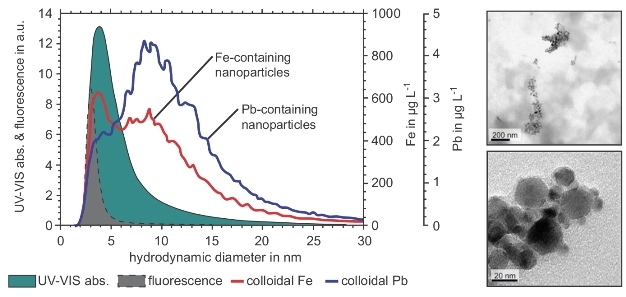
The colloidal stability of nanoparticles in the environment
The Derjaguin and Landau, Verwey and Overbeek (DLVO) theory (Derjaguijn & Landau 1941; Verwey & Overbeek 1948) describes the two opposing forces (exerted through this double layer) that act on nanoparticles that approach one another: the repulsive electrostatic potential VR, created by the ‘halo' of counter ions surrounding each particle, and the sum of attractive forces (van der Waals interactions), VA. Figure 6 shows the combined effect of forces VA and VR.
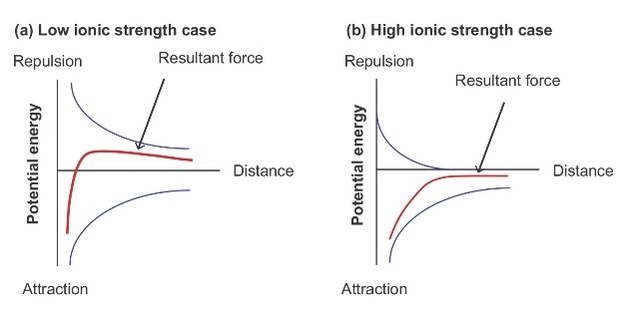
Under classical DVLO theory, if VA < VR then nanoparticles are considered kinetically stable. In reality the situation is more complex and many non-DLVO forces exist, most notably steric repulsion due to coatings such as HS, where compression of the coatings requires additional energy (Tipping & Higgins 1982). Although traditional DLVO theory can semi-quantitatively predict nanoparticle aggregation and deposition, only by including non-DLVO interactions can a complete understanding of their environmental fate be reached (Petosa et al. 2010).
Nanoparticle mobility in aqueous systems
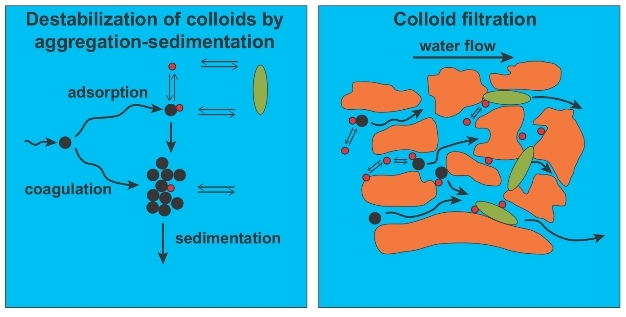
In general, the mobility of nanoparticles in surface waters is only limited by their colloidal stability. Similarly, in fractured aquifers they can move largely unhindered. However, in subsurface porous media (i.e., alluvial groundwater aquifers) nanoparticle and colloid mobility is more constrained and the movement of nanoparticles is typically predicted using colloid filtration theory (CFT) because of the greater potential for collisions with soil grains (Yao et al. 1971; Lui et al. 2009; Cullen et al. 2010). A variety of phenomena (e.g., Brownian motion, interception, sedimentation, inertia, and hydrodynamic forces) govern the number of collisions with the soil grain (Tufenkji & Elimelech 2004; Nelson & Ginn 2005). Because smaller particles experience a higher degree of random Brownian motion, this is particularly relevant for nanoparticles. A variety of retention mechanisms not considered part of CFT may also be important for the retention of nanoparticles and colloids in porous media (e.g., straining, maximum retention capacity of soil surfaces and soil surface charge heterogeneities).
Trace metal binding in nano-metal complexes
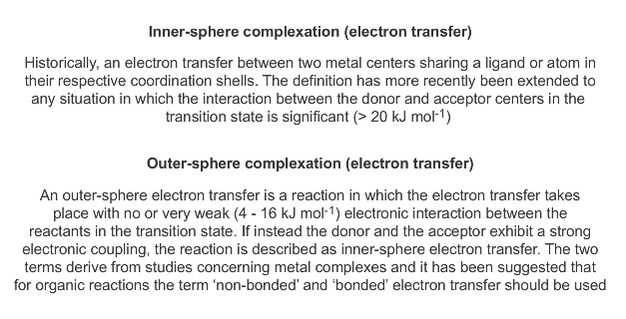
The importance of nanoparticles (over colloids in general) lies in their ability to bind large amounts of TMs, which impacts the bioavailability of vital and toxic metals in natural waters. The principles that govern these binding reactions are thought to be similar regardless of the size and chemistry of the colloids and typically occur via inner-sphere, e.g. covalent bonding, complexation reactions with surface functional groups (Box 2).
Inner-sphere complexation between metal ions and common metal oxides, which also occurs for HS and other colloids, has been shown to be the predominant mechanism of adsorption (Bargar et al. 1997; Alcacio et al. 2001) and can be conceptualized as of the type in Equation 1, where complexation of the metal ion (Mz+) follows deprotonation of a hydroxyl group (S-OH) at the aquatic particle surface:
(1) S-OH(aq) + Mz+(aq) ↔ S-OM(aq) + H+(aq)
Edge site valence and additional surface protons are omitted for convenience.
By definition, metals in inner-sphere complexes are more strongly bound, and therefore are less labile (Box 3) than metals in outer-sphere complexes (ion pairs), with implications for metal bioavailability and ecotoxicity.

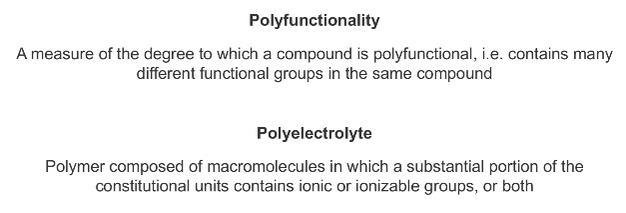
Metal binding by HS-containing nanoparticles and colloids is affected by their polyfunctionality (resulting from the presence of metal binding sites with different chemical nature) and their polyelectrolyte character (resulting from the presence of a large density of charge due to numerous dissociable sites) (Box 4). The relative importance of each of these factors depends on ambient conditions such as salt concentration, type of ions in the medium, and pH, which ultimately influence the inter- and intra-particle interactions as well as metal to HS ratio (Tipping 2001). Much less is known about metal binding by other non-humic organic nano-sized components, despite being present in large proportion within flocs and biofilms (Flemming & Wingender 2001) in freshwaters (up to 25% of DOM; Wilkinson et al. 1997) and in marine systems (up to 80% of DOM; Verdugo et al. 2004).
The ecological significance of nanoparticles
Biological availability is defined as "the extent of absorption of a substance by a living organism" (Nordberg et al. 2010). Because of the interaction of TMs with other constituents of the aquatic system (such as nanoparticles), only a fraction of the total mass of TM present interacts with organisms, and therefore, the remainder is unavailable as either a nutrient or toxin unless directly absorbed prior to dissociation. Nanoparticles and colloids regulate TM bioavailability by influencing TM speciation and other processes at the organism - environment interface (Figure 8).
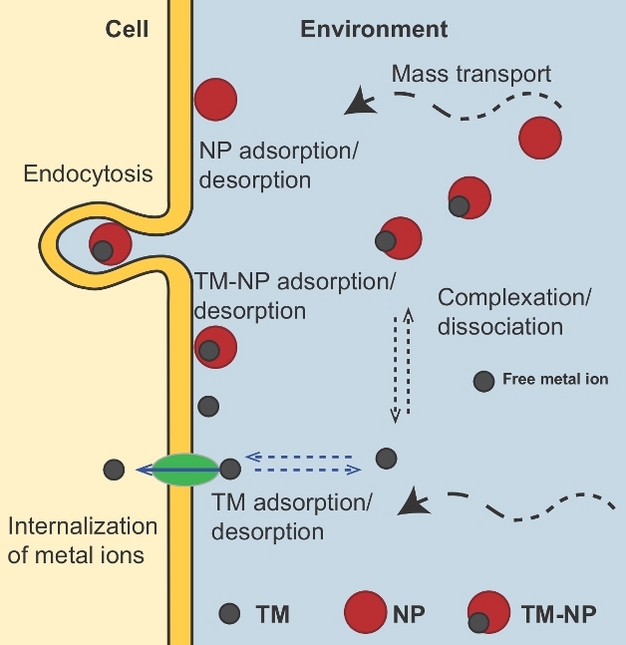
In general, colloids reduce TM bioavailability by decreasing the freely available fraction of TMs to cross the cellular membrane of an organism. Indeed, colloids of different composition have been shown to limit the bioavailability (and detrimental effects) of toxic metals (e.g., Ag, Cd, Cu, Ni and Pb) to various organisms, including bacteria, fungi, phytoplankton, daphnia and fish, in direct proportion to the free metal ion concentrations (Slaveykova & Wilkinson 2005).
Nanoparticles and colloids may also alter TM bioavailability by decreasing the mass transport towards the organism surface and lability of the associated TM. Under diffusion-limited conditions the bioavailable metal is proportional to the diffusion coefficients of the complexes and their lability (Buffle et al. 2009). Exceptions to this rule include where metallic nanoparticles (and TM-NP complexes) are an additional source of TMs for filter feeders (e.g., clams, mussels and oysters) (Luoma & Rainbow, 2005) and organisms with endocytotic capability (i.e., absorb molecules such as proteins by engulfing them) (Simkiss & Taylor 1995).
Given the large proportion of TMs bound to nanoparticles and colloids in surface waters (Lead & Wilkinson 2006), they are expected to mitigate the detrimental effects of toxic trace elements by generally decreasing their bioavailability. In addition, by regulating the bioavailability of micronutrients (Cu, Co, Fe, Mn, Mo, Ni and Zn) used in enzymatic processes (Morel & Price 2003), NPs could affect the phytoplankton biomass and diversity in the ocean and lakes (Morel & Price 2003; Sterner et al. 2004; Boyd & Ellwood 2010). Such alteration could have profound consequences for the biogeochemical cycles of C, N, Fe as well as for food-web interactions, as phytoplankton is responsible for more than 40% of the primary productivity on Earth and represents the base of the aquatic food chain. For example, the mass flux of Fe in the form of nano-clusters delivered by icebergs in the Southern Ocean has been shown to be comparable to Aeolian inputs (Raiswell et al. 2008). Changes in the mass flux of nano-Fe in response to climate changes may be an important negative feedback to warming in the Antarctic region.
Conclusion: environmental nanoparticles and geosystems
Nanogeoscience is perhaps the ultimate research frontier in geosystems. Natural nanoparticles are of central importance in the earth system: in global biogeochemical cycles, weathering, metal binding and transport, bioavailability and ecotoxicity. Furthermore, the influence of nanoparticles on the bioavailability of both nutrient and toxic elements has been a factor in the evolution and development of higher organisms, potentially buffering environmental systems against change (Tipping 2001). It is interesting therefore that manufactured nanoparticles are a focus of concern given that they are present in much lower amounts than their natural counterparts. The reason for this is that manufactured nanoparticles are made of specific structures and chemistries, as distinct from those found in nature, for which organisms may not have appropriate defense mechanisms.
Glossary
oxyhydroxides: A mixed oxide and hydroxide mineral
aluminosilicates: Minerals composed of aluminium, silicon, and oxygen, plus counter-cations
humic substances: Major components of the natural organic matter (NOM) in soil and water as well as in geologic deposits
natural organic Matter (NOM): the natural organic material present waters including both humic and non-humic fractions
aggregation: Formation of clusters in a colloidal suspension and is the most frequent mechanism of colloid destabilization in surface waters
steric: Referring to steric stabilization of a colloidal suspension by polymer-covered surfaces (e.g. NOM) or in solutions containing non-adsorbing polymer can modulate interparticle forces, producing an additional steric repulsive force
circumneutral: Having a pH between 6.5 and 7.5
electrostatic: The electric force that a particle exerts on another particle
double layer: Also called an electrical double layer (EDL), is a structure that appears on the surface of an object when it is exposed to a fluid
adsorption: Here refers to the adhesion of ions to a surface
bioavailability: The degree and rate at which a substance is absorbed into a living system
covalent: A chemical bond that involves the sharing of electron pairs between atoms
complexation: A coordination complex or metal complex, consisting of a metallic ion, surrounded by an array of bound molecules or anions
functional groups: Collections of atoms in a molecule that participate in characteristic reactions (e.g. metal coordination)
ecotoxicity: The potential for chemical stressors to affect ecosystems
endocytotic: Entry of a substance into a cell without passing through the cell membrane
References and Recommended Reading
Aitken, G.R., Hsu-Kim, H., Ryan, J.N. Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ. Sci. Technol. 45, 3196-3201 (2011).
Alcacio, T.E. et al. Molecular scale characteristics of Cu (II) bonding in goethitehumate complexes. Geochim. Cosmochim. Acta 65, 1355-1366 (2001).
Avena, M.J., Mariscal, M.M., & De Pauli, C.P. Proton binding at clay surfaces in water. Appl. Clay Sci. Clay Min. Environ. 24, 3-9 (2003).
Baalousha, M. & Lead, J.R. Characterization of Natural Aquatic Colloids (<5 nm) by Flow-Field Flow Fractionation and Atomic Force Microscopy. Environ. Sci. Technol. 41, 1111-1117 (2007).
Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci. Tot. Environ. 407, 2093-2101 (2009).
Bargar, J.R., Brown, G.E., & Parks, G.A. Surface complexation of Pb(II) at oxide-water interfaces: I. XAFS and bond-valence determination of 290 mononuclear and polynuclear Pb(II) sorption products on aluminum oxides. Geochim. Cosmochim. Acta 61, 2617-2637 (1997).
Benoit, G. Evidence of the particle concentration effect for lead and other metals in fresh waters based on ultraclean technique analyses. Geochim. Cosmochim. Acta. 59, 2677-2687 (1995).
Boyd, P. W. & Ellwood, M. J. The biogeochemical cycle of iron in the ocean. Nature Geosci. 3, 675-682 (2010).
Buffle, J. & Leppard, G.G. Characterization of aquatic colloids and macromolecules. 1. Structure and behavior of colloidal material. Environ. Sci. Technol. 29, 2169-2175 (1995).
Buffle, J., Wilkinson, K. J., Stoll, S., Filella, M. & Zhang, J. A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ. Sci. Technol. 32, 2887-2899 (1998).
Buffle, J., Wilkinson, K. J. & van Leeuwen, H. P. Chemodynamics and bioavailability in natural waters. Environ. Sci. Technol. 43, 7170-7174 (2009).
Campbell, P. G. C. Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. IUPAC Series on Analytical and Physical Chemistry of Environmental Systems 2, 45-102 (1995).
Chanudet, V. & Filella, M. The fate of inorganic colloidal particles in Lake Brienz. Aquat. Sci. 69, 199 - 211 (2007).
Chen, K., Mylon, S., Elimelech, M. Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ. Sci. Technol. 40, 1516-1523 (2006).
Cullen, E., O'Carroll, D.M., Yanful, E.K., Sleep, B. Simulation of the subsurface mobility of carbon nanoparticles at the field scale. Adv. Water Res. 33, 361-371 (2010).
Derjaguin, B.; Landau, L. (1941), "Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes", Acta Physico Chemica URSS 14, 633.
Doucet F.J., Lead J.R. & Santschi P.H. Colloid-trace element interactions in aquatic systems. In Environmental Colloids and Particles: Behaviour, Separation and Characterisation. Eds. Wilkinson, K. & Lead, J.R. (Chichester: John Wiley & Sons Ltd., 2007). 95-157.
Duval, J.F.L., Wilkinson, K.J., van Leeuwen, H.P., Buffle, J. Humic substances are soft and permeable: evidence from their electrophoretic mobilities. Environ. Sci. Technol. 39, 6435-6445 (2005).
Filella, M. NOM site binding heterogeneity in natural waters: Discrete approaches. J. Mol. Liq. 143, 42-51 (2008).
Flemming H.C.Wingender J. Relevance of microbial extracellular polymeric substances (EPSs). Part 1. Structural and ecological aspects. Water Sci. Technol. 43, 1-8 (2001).
Gaillard, J.F. Probing environmental colloids and particles with x-rays. In Environmental Colloids and Particles: Behaviour, Separation and Characterisation. Eds. Wilkinson, K. & Lead, J.R. (Chichester: John Wiley & Sons Ltd., 2007). 613-616.
Gibson, C.T., Turner, I.J., Roberts, C.J., & Lead, J.R. Quantifying the dimensions of nanoscale organic surface layers in natural waters. Environ. Sci. Technol. 41, 1339-1344 (2007).
Grasso, D., Subramaniam, K., Butkus,M., K Strevett, Bergendahl, J. A review of non-DLVO interactions in environmental colloidal systems. Rev. Environ. Sci. Biotech. 1, 17-38 (2002).
Hartland, A. et al. From soil to cave: Transport of trace metals by natural organic matter in karst dripwaters. Chem. Geol. 304-305, 68-82 (2012).
Hartland, A., Fairchild, I.J., Lead, J.R., Zhang, H. & Baalousha, M. Size, speciation and lability of NOM-metal complexes in hyperalkaline cave dripwater. Geochim. Cosmochim. Acta 75, 7533-7551 (2011).
Hassler, C. S., Alasonati, E., Nichols, C. A. M. & Slaveykova, V. I. Exopolysaccharides produced by bacteria isolated from the pelagic Southern Ocean - Role in Fe binding, chemical reactivity, and bioavailability. Mar. Chem. 123, 88-98 (2011).
Hochella, M.F. There's plenty of room at the bottom: Nanoscience in geochemistry. Geochim. Cosmochim. Acta 66, 735 (2002).
Howard, A.G. Aquatic Environmental Chemistry. (Oxford Chemistry Primers).
Jarvie, H.P. et al. Role of riverine colloids in macronutrient and metal partitioning and transport, along an upland-lowland land-use continuum, under low-flow conditions. Sci. Tot. Environ. 434, 171-185 (2012).
Lead, J. R. & Wilkinson, K. J. Aquatic colloids and nanoparticles: Current knowledge and future trends. Environ. Chem. 3, 159-171 (2006).
Lead, J.R., Hamilton-Taylor, J., Davison, W. & Harper, M. Trace metal sorption by natural particles and coarse colloids. Geochim. Cosmochim. Acta 63, 1661-1670 (1999).
Liu, X., O'Carroll, D.M., Petersen, E.J., Huang, Q. and Anderson, L.C. Mobility of multi-walled carbon nanotubes in porous media. Environ. Sci. Technol. 43, 8153-8158 (2009).
Lofts, S. & Tipping, E. Assessing WHAM/Model VII against field measurements of free metal ion concentrations: Model performance and the role of uncertainty in parameters and inputs. Env. Chem. 8, 501-516 (2011).
Luoma, S. N. & Rainbow, P. S. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 39, 1921-1931 (2005).
Lyven, B., Hassellov, M., Turner, D.R., Haraldsson, C., & Andersson, K. Competition between iron- and carbon-based colloidal carriers for trace metals in a freshwater assessed using flow field-flow fractionation coupled to ICPMS. Geochim. Cosmochim. Acta 67, 3791 (2003).
Madden, A.S., Hochella Jr., M.F., Luxton, T.P. Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim. Cosmochim. Acta. 70, 4095-4104 (2006).
McDonald, S., Bishop, A.G., Prenzler, P.D., & Robards, K. Analytical chemistry of freshwater humic substances. Anal. Chim. Acta 527, 105- 124 (2004).
Morel, F. M. M. & Price, N. M. The biogeochemical cycles of trace metals in the oceans. Science 300, 944-947 (2003).
Nelson, K.E., & Ginn, T.R. Colloid filtration theory and the Happel sphere-in-cell model revisited with direct numerical simulation of colloids. Langmuir 21, 2173-2184 (2005).
Nordberg, M., Duffus, J. H. & Templeton, D. M. Explanatory dictionary of key terms in toxicology: Part II (IUPAC Recommendations 2010). Pure App. Chem. 82, 679-751 (2010).
Petosa, A.R., Jaisi, D.P., Quevedo, I.R., Elimelech, M., Tufenkji, N. Aggregation and Deposition of Engineered Nanomaterials in Aquatic Environments: Role of Physicochemical Interactions. Environ. Sci. Technol. 44, 6532-6549 (2010).
Raiswell, R., Benning, L. G., Tranter, M., & Tulaczyk, S. Bioavailable iron in the Southern Ocean: the significance of the iceberg conveyor belt. Geochemical transactions, 9(7) (2008).
Santschi, P.H., Roberts, K.A. & Guo, L. Organic nature of colloidal actinides transported in surface water environments. Environ. Sci. Technol. 36, 3711-3719 (2002).
Simkiss, K. & Taylor, M. G. in Metal speciation and bioavailability in aquatic systems, IUPAC series on analytical and physical chemistry of environmental systems Vol. 3 (Eds A. Tessier & D.R. Turner) 2-44 (John Wiley, 1995).
Slaveykova, V. I. & Wilkinson, K. J. Predicting the bioavailability of metals and metal complexes: Critical review of the biotic ligand model. Environ. Chem. 2, 9-24 (2005).
Slaveykova, V. I., Wilkinson, K. J., Ceresa, A. & Pretsch, E. Role of fulvic acid on lead bioaccumulation by Chlorella kesslerii. Environ. Sci. Technol. 37, 1114-1121, (2003).
Steinberg, C. E. W., Meinelt, T., Timofeyev, M. A., Bittner, M. & Menzel, R. Humic substances part 2: Interactions with organisms. Envron. Sci. Poll. Res. 15, 128-135 (2008).
Sterner, R. W. et al. Phosphorus and trace metal limitation of algae and bacteria in Lake Superior Limnol. Oceanogr. 49, 495-507 (2004).
Stolpe, B. & Hassellöv, M. Changes in size distribution of fresh water nanoscale colloidal matter and associated elements on mixing with seawater. Geochim. Cosmochim. Acta 71, 3292-3301 (2007).
Sutton, R. & Sposito, G. Molecular structure in humic substances: the new view. Environ. Sci. Technol. 39, 9009-9015 (2005).
Tipping, E. & Higgins, D.C. The effect of adsorbed humic substances on the colloid stability of hematite particles. Coll. Surf. 5, 85-92 (1982).
Tipping, E. Cation binding by humic substances. Cambridge: University Press (2001).
Tufenkji, N., & Elimelech M. Deviation from classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir 20, 10818-10828 (2004).
Verdugo P, Alldredge AL, Azam F, Kirchmand DL, Passow U, Santschi PH. The oceanic gel phase: a bridge in the DOM-POM continuum. Mar. Chem. 92, 67-85 (2004).
Verwey, E. J. W.; Overbeek, J. Th. G. Theory of the stability of lyophobic colloids, Amsterdam: Elsevier (1948).
Warwick, P., Hall, A., Pashley, V., & Bryan, N. Investigation of the permeability of humic molecules using zeta potential measurements. Chemosphere 45, 303-307 (2001).
Wigginton N. S., Haus K. L., Hochella, M. F. Jr. Aquatic environmental nanoparticles. J. Env. Monit. 9, 1306-1316 (2007).
Wilkinson KJ, Joz-Roland A, Buffle J. 1997. Different roles of pedogenic fulvic acids and aquagenic biopolymers on colloid aggregation and stability in freshwaters. Limnol. Oceanogr. 42, 1714-1724 (1997).
Wilkinson, K. J. & Buffle, J. Critical evaluation of physicochemical parameters and Processes for modelling the biological uptake of trace metals in environmental (aquatic) systems. Physicochemical Kinetics and transport at Biointerfaces. Eds. van Leewen, H. P. & Koester, W. (Chichester: John Wiley & Sons, 2004). 447-533.
Wilkinson, K.J. & Reinhardt, A. Contrasting roles of natural organic matter on colloidal stabilization and flocculation in freshwaters. In Flocculation in Natural and Engineered Environmental Systems. Eds. Droppo, I. G., Leppard, G. G., Liss, S. N., & Milligan, T. (Boca Raton: CRC Press, 2005).
Yao, K. M., Habibian, M. T. & O'Melia, C. R. Water and waste water filtration: concepts and applications. Environ. Sci. Technol. 5, 1105-1112 (1971).