Abstract
Schizophrenia patients exhibit deficits in signaling of the M1 subtype of muscarinic acetylcholine receptor (mAChR) in the prefrontal cortex (PFC) and also display impaired cortical long-term depression (LTD). We report that selective activation of the M1 mAChR subtype induces LTD in PFC and that this response is completely lost after repeated administration of phencyclidine (PCP), a mouse model of schizophrenia. Furthermore, discovery of a novel, systemically active M1 positive allosteric modulator (PAM), VU0453595, allowed us to evaluate the impact of selective potentiation of M1 on induction of LTD and behavioral deficits in PCP-treated mice. Interestingly, VU0453595 fully restored impaired LTD as well as deficits in cognitive function and social interaction in these mice. These results provide critical new insights into synaptic changes that may contribute to behavioral deficits in this mouse model and support a role for selective M1 PAMs as a novel approach for the treatment of schizophrenia.
Similar content being viewed by others
INTRODUCTION
Schizophrenia is a chronic debilitating psychiatric disorder that is characterized by positive (hallucinations, delusions), negative (affective flattening, social withdrawal), and cognitive symptoms (working memory and attentional deficits) (Lewis and Lieberman, 2000). A strong body of evidence has implicated a dysfunction of prefrontal cortex (PFC) as a key component of the pathophysiology underlying symptomology of schizophrenia. These studies have primarily demonstrated task-related hypofrontality in patients as well as animal models (for review, see Manoach, 2003). However, a recent and rapidly emerging body of clinical and preclinical studies also suggest that excessive activation of the PFC by subcortical excitatory projections may contribute to the negative symptoms and cognitive deficits observed in schizophrenia (Jodo, 2013; Manoach, 2003; Woodward et al, 2013). It is possible that this excessive activation of the PFC may be due to an imbalance in the two common forms of plasticity in PFC, namely, long-term potentiation (LTP) and long-term depression (LTD). Indeed, recent clinical literature suggests that there are deficits in LTD in patients diagnosed with schizophrenia (Hasan et al, 2013; Strube et al, 2014). Furthermore, a common preclinical model of schizophrenia involving transient blockade of N-methyl-D-aspartate receptors (NMDARs) with repeated administration of NMDAR antagonists during a critical period of adolescent development induces profound and lasting deficits in LTD at synapses from the hippocampus to PFC (Ghoshal and Conn, 2015; Thomases et al, 2014). This has been postulated to contribute to the increased activity of PFC neurons observed following phencyclidine (PCP) treatment in rodents (Katayama et al, 2007; Suzuki et al, 2002; Thomases et al, 2014; Wang et al, 2007) as well as the subsequent behavioral changes associated with negative and cognitive symptoms in the disorder (Neill et al, 2010, 2014).
Muscarinic acetylcholine receptors (mAChRs) play important roles in regulating synaptic plasticity in the PFC and activation of mAChRs induces LTD at the hippocampo-PFC synapse (Lopes-Aguiar et al, 2013; Parent et al, 2010; Wang and Yuan, 2009). Interestingly, multiple studies suggest that cholinergic signaling is disrupted in schizophrenia patients and in animal models of schizophrenia (Berman et al, 2007; Dean et al, 2002; Scarr and Dean, 2009b; Zavitsanou et al, 2004). If these changes in cholinergic signaling lead to impairments in muscarinic LTD (mLTD), this could contribute to the underlying pathophysiology in PFC function. Importantly, mAChR antagonists exacerbate cognitive deficits and negative symptoms in schizophrenia patients (Veselinovic et al, 2014) and xanomeline, a mAChR agonist, reduces all symptom clusters in schizophrenia patients (Shekhar et al, 2008) and corresponding animal models (Barak and Weiner, 2011; Stanhope et al, 2001). These data are consistent with the hypothesis that changes in mAChR signaling could contribute to the symptoms observed in schizophrenia patients. Of the five mAChR subtypes (M1–M5), M1 is the predominant mAChR subtype expressed in the PFC (Levey et al, 1991) and likely participates in induction of mLTD (Caruana et al, 2011) and other physiological responses that are important for PFC-dependent cognitive functions (Digby et al, 2012; Shirey et al, 2009). We now report discovery and optimization of VU0453595 as a novel, highly selective positive allosteric modulator (PAM) for M1 that provides excellent pharmacokinetic properties and brain exposure in mice after systemic administration. We used VU0453595, along with M1-knockout (KO) mice and mice selectively expressing channelrhodopsin in cholinergic neurons, to show that mLTD in the PFC is mediated exclusively by the M1 mAChR subtype. Interestingly, daily administration of the NMDAR antagonist PCP to juvenile mice led to a complete and lasting loss of M1-mediated LTD in PFC slices. Incubation of PFC slices with the M1-selective PAM restored the deficits in mLTD and systemic administration of VU0453595 reversed deficits in social interaction and cognitive function observed in this rodent model of schizophrenia. These data provide new insights into the functional impact of changes in M1 signaling in a model of schizophrenia and raise the exciting possibility that highly selective M1 PAMs may provide a novel approach for reducing cognitive deficits and negative symptoms associated with changes in cortical plasticity in schizophrenic patients.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Vanderbilt University Medical Center Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) were used in electrophysiology and behavioral studies (8–9 weeks old). The 8–9-week-old male M1-KO (Hashimoto et al, 2008) and B6.Cg-Tg(Chat-COP4*H134R/EYFP,Slc18a3)5Gfng/J (Jackson Laboratories; Santini et al, 2013) mice (both C57BL6 background) were also utilized for electrophysiology studies.
Phencyclidine Repeat Dosing
The 6–7-week-old male C57Bl/6 mice (Jackson Laboratories) were used for repeated PCP dosing studies. Mice were injected subcutaneously (s.c.) with 10 mg/kg of PCP (Sigma, St Louis, MO) or vehicle (saline) at a volume of 10 ml/kg once daily for 7 consecutive days. The electrophysiology or behavioral tasks were performed following a 7-day washout period. Separate groups of mice were used for each behavioral task and electrophysiology studies.
Extracellular Field Potential Recordings
Extracellular field potential recordings were performed with 8–9-week-old male C57BL6/J mice (Jackson Laboratories) mice using coronal slices (400 μm) containing prelimbic PFC. Paired-pulse field excitatory postsynaptic potentials (fEPSPs) were recorded from the layer V of the prelimbic prefrontal cortex and evoked by paired electrical stimulus pulses (100 μs duration, every 20 s; interpulse interval of 50 ms) of the superficial layers II–III, delivered through a concentric bipolar stimulating electrode. For studies involving optical stimulation, blue light (470 nm) was delivered using a High Power LED (Thorlabs, Newton, NJ). Detailed methods are described in Supplementary Methods.
Whole-Cell Patch-Clamp Recordings
Whole-cell patch-clamp recordings were performed using coronal slices (300 μm) prepared from 8–9-week-old male C57BL6/J mice (Jackson Laboratories) and a K-gluconate-based intracellular solution as described previously (Shirey et al, 2009). Spontaneous EPSCs were recorded at a holding potential of −70 mV that is close to the reversal potential of chloride ions; therefore, GABAA receptor-mediated inhibitory currents were undetectable under these conditions. The interevent intervals of sEPSCs from 2 min episodes during baseline and drug application were used to generate cumulative probability plots. The interevent intervals from each experiment were then expressed as frequency and the mean values from the 2 min episodes were grouped and compared. Inward current data analysis was performed using Clampfit software where the peak amplitude of the inward current was measured (v10.2, Molecular Devices, Sunnyvale, CA). Detailed methods are described in Supplementary Methods.
Social Interaction Assay
Mice were allowed to habituate to a plexiglass arena consisting of white walls and a clear lid (36 × 26 × 18 cm) for 60 min. Mice were then injected with vehicle (20% β-cyclodextrin (BCD)), 1, 3, or 10 mg/kg of VU0453595 (intraperitoneal (i.p.); 10 ml/kg) and placed back into the testing arena. Following a 30-min pretreatment, an age/weight-matched noncagemate (intruder) mouse was placed in the testing arena with the test mouse and their interaction behavior was recorded for 5 min. The amount of time spent in social contact (active contact, social sniffing, and social follow) initiated by the test mouse was recorded by an observer blinded to experimental condition.
Novel Object Recognition Task
Mice were habituated in an empty novel object recognition (NOR) arena (consisting of dark-colored plexiglass box (32 × 32 × 40 cm) for 10 min. Approximately 24 h later, the mice were injected with vehicle (20% BCD), 1, 3, or 10 mg/kg of VU0453595 (i.p.; 10 ml/kg) and placed back into their home cage for 30 min. Mice were then placed back into the NOR arena containing 2 identical objects for 10 min. Following the exposure period, mice were placed back into their home cages for 1 h. The mice were then returned to the arena in which one of the previously exposed (familiar) objects was replaced by a novel object. The mice were video recorded for 10 min while they explored the objects. Time spent exploring each object was scored by an observer blinded to the experimental conditions and the recognition index was calculated as [(time spent exploring novel object)−(time spent exploring familiar object)]/total time exploring objects.
Statistical Analysis
All statistical analyses were performed in GraphPad Prism 5 (GraphPad, San Diego, CA). The effects of various pharmacological and genetic conditions on LTD and behavioral effects were compared using one-way analysis of variance (ANOVA) with Dunnett’s post hoc tests unless otherwise mentioned. Paired pulse ratios (PPRs) were compared across groups using paired t-test.
RESULTS
Muscarinic LTD in Prefrontal Cortex Is Solely Mediated by the M1 mAChR Subtype
The fEPSPs were recorded from layer V neurons while stimulating the superficial layer II–III neurons of prelimbic PFC using a bipolar stimulating electrode (Figure 1a). Stimulation with paired pulses led to reliable fEPSPs that exhibited a PPR ranging from 0.9 to 1.5. Bath application of 50 μM carbachol (CCh) for 10 min led to a robust short-term depression followed by moderate LTD (67.7±6.1% baseline fEPSP) that lasted for at least 60 min following CCh application (Figure 1b and f) and accompanied by a significant increase in the PPR when compared with the baseline (120.0±4.8%; p=0.0021; Figure 1c and g). This 50 μM CCh-induced LTD was a saturated form of LTD in mouse PFC as higher concentration of CCh (100 μM) produced an LTD of similar magnitude (69.6±5.0% baseline fEPSP; Figure 1f, white bar). Bath application of the orthosteric M1-selective antagonist (Sheffler et al, 2009) VU0255035 (10 μM) 10 min before and during CCh application led to a complete abolition of the CCh-induced mLTD (99.7±11.7%), although the short-term depression in response remained unaffected (Figure 1d and f). Finally, application of 50 μM CCh in PFC slices prepared from M1-KO mice led to a short-term depression of baseline response but did not induce mLTD (96.0±4.0%; Figure 1e and f). There was a significant difference in the magnitude of mLTD between the experimental groups (p=0.0077; F=5.548; R2=0.4947), with the magnitude of mLTD observed with 50 μM CCh in WT mice significantly more from the VU0255035+50 μM CCh condition (p<0.05) or in the M1-KO mice (p<0.05) but not different from the 100 μM CCh condition (p>0.05; Figure 1f). No increase in PPR was observed in conditions where mLTD was not observed (p>0.05; 90.5±4.4% (VU0255035+50 μM CCh) and p>0.05; 105.9±3.5% (M1-KO); Figure 1g). Taken together, these data suggest that CCh-induced mLTD in the PFC is characterized by an increase in PPR and requires activation of the M1 mAChR subtype.
Muscarinic LTD (mLTD) in PFC is mediated by the M1 muscarinic receptor subtype. (a) The fEPSPs were recorded from layer V of the mouse PFC in response to paired stimulus pulses in the superficial layers II–III. The right panel shows an example trace from a single trial showing typical fEPSPs in response to a paired pulse. (b) The mLTD (shaded gray area) in the PFC of WT drug-naive mice was induced by bath application of 50 μM CCh for 10 min (solid line). Inset shows sample trace: black trace, baseline; gray trace, 60 min following CCh application. (c) Significant increase in the PPR was observed during mLTD (late phase: 55–60 min following CCh application) when compared with that during the baseline. (d) The 50 μM CCh-induced mLTD is blocked by pretreatment of 10 μM M1 orthosteric antagonist VU0255035 (gray shaded area; light gray sample trace) for 10 min followed by coapplication with 50 μM CCh (dashed line). (e) The 50 μM CCh-induced mLTD is absent in the PFC of M1-KO mice (gray shaded area; dark gray sample trace). (f) Quantification of mLTD (normalized fEPSP slopes) in the different experimental groups showing that there were significant differences in the magnitude of mLTD observed with 50 μM CCh (n=6) in WT mice when compared with the one observed with M1 antagonist VU0255035+50 μM CCh (n=5) or in the M1-KO mice (n=6). The mLTD observed with 100 μM CCh (n=4) was of similar magnitude to that of 50 μM CCh with no significant differences observed between them (p>0.05). (g) Quantification of PPRs during the late phase (normalized to baseline) from each experimental group showing that there was an overall significant increase in PPR during the late phase in WT slices with 50 μM CCh treatment that was not observed in the M1 antagonist VU0255035+50 μM CCh condition (p>0.05) or in M1-KO (p>0.05) mice. **P<0.01. Error bars denote SEM. CCh, carbachol; PFC, prefrontal cortex; PPR, paired pulse ratio; Rec., recording pipette; Stim., stimulating electrode. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Novel Selective M1 PAM, VU0453595, Potentiates M1-Mediated mLTD
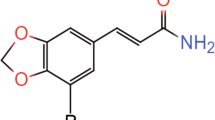
We developed a novel and highly selective M1 PAM 6-(2-Fluoro-4-(1-methyl-H-pyrazol-4-yl)benzyl)-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one (VU0453595) that exhibits excellent in vitro and in vivo properties that are described in detail in the Supplementary Materials (Supplementary Figure S1 and Supplementary Tables S1 and S2). VU0453595 belongs to a series of isoindolinone M1-selective PAMs (see Supplementary Methods for details on chemical synthesis of VU0453595), has no off-target activity (Supplementary Table S3), and shows no adverse effect liabilities that were associated with some previous-generation M1 PAMs (Supplementary Figure S2 and Supplementary Table S4). We tested the hypothesis that VU0453595 can potentiate M1-mediated mLTD. Bath application of 10 μM CCh for 10 min led to a robust acute depression followed by negligible mLTD (93.9±2.0%; Figure 2a and d) and no increase in PPR (p>0.05; 99.9±3.8%; Figure 2e) in WT mice. However, pretreatment with VU0453595 (10 μM, 10 min) before and during application of CCh (10 μM, 10 min) led to an induction of robust mLTD (66.6±6.5%; Figure 2b and d) with an increase in PPR (p<0.05; 134.1±14.6%; Figure 2e). When applied alone, the M1 PAM VU0453595 (10 μM, 20 min) failed to produce any mLTD measured 60–65 min following application of the compound (96.3±4.2%; Supplementary Figure S3a and c) in WT PFC slices. Finally, VU0453595 potentiation of subthreshold CCh-induced mLTD (95.6±2.4% baseline fEPSP; Figure 2c and d) and increase in PPR (p>0.05; 101.1±6.4%; Figure 2e) were absent in PFC slices from M1-KO mice. There was a significant difference in the magnitude of mLTD observed between groups (p=0.0002; F=15.29; R2=0.6709) with a significant difference between 10 μM CCh alone and the VU0453595+10 μM CCh condition (p<0.001) in WT mice. In contrast, there was no significant difference in mLTD between 10 μM CCh alone in WT mice and the VU0453595+10 μM CCh condition in M1-KO mice (p<0.05), indicating that VU0453595 can potentiate a threshold form of mLTD specifically via activation of M1 receptors (Figure 2d).
The M1 PAM VU0453595 potentiates muscarinic LTD by activating M1 receptors. (a) Time-course graph showing that, unlike 50 μM CCh, application of 10 μM CCh for 10 min (solid line) led to a negligible mLTD (shaded gray area; gray sample trace). (b) M1 PAM VU0453595 potentiated the threshold 10 μM CCh-mediated response as pretreatment (dashed line) with VU0453595 led to a robust mLTD (shaded gray area; light gray sample trace). (c) Under similar conditions, VU0453595 failed to potentiate threshold 10 μM CCh-mediated response in the PFC of M1-KO mice (shaded gray area; dark gray sample trace). (d) Quantification of mLTD (normalized fEPSP slopes) in the different experimental groups. There was a significant difference in the magnitude of mLTD observed with 10 μM CCh alone (n=6) in WT mice when compared with the VU0453595+10 μM CCh condition (n=6). In contrast, there was no significant difference in mLTD between 10 μM CCh alone in WT mice and the VU0453595+10 μM CCh condition in M1-KO mice (n=6). (e) Quantification of PPRs during the late phase (normalized to baseline) from each experimental group. An increase in PPR during the late phase is observed in the WT, VU0453595+10 μM CCh condition but is not observed in slices treated with 10 μM CCh alone or the in M1-KO slices. *P<0.05; ***P<0.001. Error bars denote SEM. A full color version of this figure is available at the Neuropsychopharmacology journal online.
To investigate whether M1-dependent mLTD can be induced with physiological concentrations of ACh, we next determined the effect of VU0453595 on LTD induced by optical stimulation of endogenous ACh release. Optical stimulation (15 min at 2 Hz) induced release of endogenous ACh in ChAT-ChR2-YFP BAC transgenic mice and led to a small but significant mLTD (87.1±2.5%; Supplementary Figure S4; paired t-test; p=0.0109 when compared with baseline). However, pretreatment with 10 μM VU0453595 for 5 min before and during optical stimulation (15 min at 2 Hz) induced a robust mLTD (67.4±3.9%; Supplementary Figure S4). This VU0453595-induced potentiation of optically induced mLTD was completely blocked by pretreatment with the M1 antagonist VU0255035 (10 μM; 89.9±3.8%; Supplementary Figure S4). There was a significant difference in the magnitude of optically induced mLTD observed between groups (p=0.0026; F=12.40; R2=0.7337), where the group with combined M1 PAM VU0453595 and optical stimulation displayed significantly more mLTD than optical stimulation alone or the M1 antagonist VU0255035+optical stimulation conditions (p<0.05; Supplementary Figure S4b). In addition, similar to the WT mice, application of the M1 PAM VU0453595 (10 μM) alone for 20 min in ChAT-ChR2-YFP BAC transgenic mouse PFC slices failed to produce any mLTD (n=3; 103.6±9.6%). These results indicate that the M1 PAM VU0453595 can potentiate both exogenous agonist-induced mLTD and mLTD induced by endogenous ACh released from local cholinergic terminals in an M1-dependent manner.
Repeated PCP Treatment Induces a Deficit in mLTD That Can be Reversed by VU0453595
PCP (10 mg/kg; s.c.) was injected once daily in 6–7-week-old mice for 7 consecutive days followed by a 7-day washout period without PCP administration (Figure 3a). Repeated PCP exposure did not alter the input–output relationship (two-way ANOVA; p=0.3772 for interaction; p=0.06 for treatment) or the PPR (two-way ANOVA; p=0.9738 for interaction; p=0.4936 for treatment) observed in the PFC slices (Figure 3b). However, 50 μM CCh failed to induce mLTD (101.4±7.6%; Figure 3c and e) or increase in PPR (p>0.05; 103.3±8.0%; Figure 3f) in the PCP-treated mice. Similarly, 100 μM CCh also failed to induce any form of mLTD in these PCP-treated mice (n=5; 97.1±6.3%). In contrast, a previously reported form of mGlu2/3 agonist LY379268-mediated glutamatergic LTD (Walker et al, 2015) was unaffected in the PCP-treated mice (Supplementary Figure S5a and b). This indicated that the deficit in mLTD is specific to deficits in muscarinic plasticity.
Muscarinic LTD is compromised following repeated PCP treatment but can be rescued by the M1 PAM VU0453595. (a) Dosing paradigm for repeated PCP experiments in 6–7-week-old C57Bl6 mice. Slice electrophysiology experiments were performed on day 8 or 9 of wash-out. (b) No significant differences were observed in the input–output relationship (left) and PPR for different stimulus intensities (right) for fEPSPs recorded from the layer V PFC between the drug-naive (n=22) and the PCP-treated animals (n=14). (c) Unlike in drug-naive animals as seen in Figure 1b, 50 μM CCh application for 10 min (solid line) in PCP-treated mice failed to induce mLTD in layer V PFC (shaded gray area; gray sample trace). (d) Pretreatment of 10 μM VU0453595 (dashed line) before and during 50 μM CCh application rescued mLTD in the PCP-treated mice and led to robust depression PFC fEPSP (shaded gray area; black sample trace). (e) The average magnitude of mLTD observed in the PCP-treated experimental groups was quantified and compared along with that of drug-naive 50 μM CCh group (from Figure 1f). There was a significant loss of mLTD after repeated PCP treatment (n=6) when compared with the mLTD magnitude in drug-naive mice (p<0.01). Interestingly, bath application of VU0453595 before 50 μM CCh in PCP-treated mouse slices (n=6) significantly increased the magnitude of mLTD compared with 50 μM CCh treatment alone, indicating that the M1-selective PAM can rescue deficits in muscarinic plasticity after repeated PCP treatment. (f) Quantification of normalized PPR during mLTD from each experimental group. Consistent with the hypothesis, a lack of mLTD with 50 μM CCh in the PCP-treated mice is correlated with a lack of a significant increase in PPR during the late phase in these mice. On the other hand, rescuing mLTD by VU0453595 in the PCP-treated mice is paralleled with a significant increase in PPR during the late phase. **P<0.01. Error bars denote SEM. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Perfusion of brain slices from PCP-treated mice with VU0453595 for 10 min before and during application of 50 μM CCh completely rescued deficits in mLTD (69.0±5.3%; Figure 3d and e) and the accompanying increase in the PPR (p<0.01; 127.0±8.2%; Figure 3f) in PFC slices from PCP-treated mice. There was a significant difference in the magnitude of mLTD observed between groups (p=0.0027; F=8.969; R2=0.5446). Thus, a significant loss of mLTD after repeated PCP treatment when compared with the mLTD magnitude in drug- naive mice (p<0.01) was significantly reversed by VU0453595 pretreatment in PCP-treated mouse slices (p<0.01). However, application of VU0453595 (10 μM, 20 min) by itself failed to produce any mLTD in the PCP-treated mouse PFC (95.1±1.8%; Supplementary Figure S3b and c). Thus, acute potentiation of M1 signaling is sufficient to rescue deficits in CCh-induced mLTD observed after repeated NMDAR blockade in juvenile mice.
Repeated PCP Treatment Does Not Alter General M1 Function in PFC
CCh-induced increase in sEPSC frequency and inward currents are known to be mediated by M1 mAChR subtype, with a maximal increase observed with a 100 μM CCh concentration (Shirey et al, 2009). Bath application of 100 μM CCh (a concentration that produced mLTD in drug-naive mice but failed to produce mLTD in PCP-treated mice) led to a transient increase in the sEPSC frequency of similar magnitude in pyramidal neurons of both drug-naive (447.3±125.0% baseline) and PCP-treated mice (547.6±71.7%; p=0.47; Figure 4a) when measured at a holding potential of −70 mV. This was near the calculated reversal potential for chloride ions (−69 mV) where the contribution of GABA-mediated currents is negligible. We observed that application of 10 μM CNQX (AMPA receptor antagonist) combined with 50 μM AP-V (NMDA receptor antagonist) completely abolished all detectable synaptic events, showing that they were primarily glutamatergic in nature. Application of 100 μM CCh in the presence of CNQX and AP-V also failed to increase the frequency of the sEPSCs, indicating that the spontaneous events were primarily excitatory in nature.
The general function of M1 receptors in mouse PFC is intact after repeated PCP treatment. (a) Whole-cell recordings from pyramidal neurons (regular spiking firing) at −70 mV were performed in layer V of the prelimbic prefrontal mouse cortex. A sample trace and the cumulative probability of interevent intervals for a typical cell are shown in the left during baseline, drug-add, and washout. In both drug-naive and PCP-treated mice, application of 100 μM CCh leads to an increased frequency of sEPSCs as seen in a sample trace and the cumulative probability of interevent interval that comes back to baseline levels after CCh washes out. Quantification of the effect of 100 μM CCh (normalized frequency of sEPSCs) in both experimental groups is shown in the lower right, showing no significant difference in the magnitude of increase in frequency after 100 μM CCh between them (drug naive: n=4; PCP treated: n=7). (b) To evaluate the effect of 100 μM CCh on postsynaptic M1 receptors in mouse PFC, whole-cell recordings were performed in pyramidal cells in the presence of 0.5–1 μM TTX at −70 mV. In both PCP-treated and drug-naive mice, application of 100 μM CCh for 3 min (solid red line) leads to a generation of an inward current of similar magnitude as quantified on the right (example traces on the left), showing no significant differences (drug naive: n=4; PCP treated: n=7). Pretreatment with the M1 antagonist VU0255035 (10 μM) blocked the generation of inward current in the PCP-treated mice. Thus, the magnitude of inward current observed in the PCP-treated mice with 100 μM CCh alone is significantly different than what is observed after pretreatment with M1 antagonist VU0255035 (n=3). ***P<0.001. Error bars denote SEM. A full color version of this figure is available at the Neuropsychopharmacology journal online.
Furthermore, as previously reported, 100 μM CCh, in the presence of 0.5–1 μM tetrodotoxin (TTX), induced inward currents in layer V PFC pyramidal neurons (voltage clamped at −70 mV) that were similar in magnitude in drug-naive (Δ picoAmpere (pA): 34.2±3.0) and PCP-treated (ΔpA: 43.5±4.0) mice (p=0.14). The orthosteric M1 antagonist VU0255035 for 5 min before and during application of 100 μM CCh blocked the CCh-induced inward current (ΔpA: 3.7±4.7) in the PFC slices from PCP-treated mice (p=0.0001; Figure 4b). Thus, repeated administration of PCP in juvenile animals does not lead to generalized deficits in M1 function in PFC.
Repeated PCP Administration Induces Behavioral Deficits That Can be Reversed by VU0453595
We tested the performance of the PCP-treated mice in a social interaction assay (representing a component of the negative symptom domain) and a NOR task (representing one aspect of cognitive function) 1 week after the last administration of PCP (Figure 5a). In the social interaction task, on average, saline-treated mice spent 78.0±6.9 s interacting with the intruder mouse, whereas PCP-treated mice spent significantly less time in social contact (53.5±4.3 s; p<0.05). An acute dose of VU0453595 (1, 3, and 10 mg/kg, i.p.; Figure 5b) administered 30 min before the social interaction test rescued deficits in social interaction observed in PCP-treated mice, with no significant differences observed between the VU0453595+PCP-treated and the vehicle-treated control group (p>0.05; Figure 5b). A one-way ANOVA revealed a significant effect of dose on time spent in active contact (p=0.009; F=3.3; R2=0.1630).
Behavioral deficits observed after repeated PCP exposure in mice can be rescued by an acute treatment of M1 PAM VU0453595. (a) Dosing paradigm for repeated PCP experiments for behavioral experiments. Social interaction studies were then performed on day 8 of washout, whereas novel object recognition (NOR) studies were carried out on day 8 and 9 of washout. (b) Sketch of experimental design for the social interaction studies. PCP-treated mice (n=15) spent significantly less of time in active contact when compared with the vehicle-treated, drug-naive animals (n=15). Acute treatment of 10 mg/kg VU0453595 in the vehicle group (n=16) did not significantly alter the performance in this task, but 1, 3, and 10 mg/kg (n=15) dose of VU0453595 (i.p.) in the PCP-treated mice significantly rescued the behavioral deficits, as no significant difference was observed compared with the vehicle-treated control group. (c) Schematic describing the NOR task employed in this study. PCP-treated mice (n=13) had a significantly lower recognition index when compared with the vehicle-treated animals (n=16). Acute treatment of 10 mg/kg VU0453595 in the vehicle group (n=14) did not significantly alter the performance in this task but acute treatment of VU0453595 (i.p.) in the PCP-treated mice restored their recognition index to control levels as no significant difference was observed between the 1 mg/kg (n=13), 3 mg/kg (n=14), and 10 mg/kg (n=15) VU0453595-administered PCP-treated mice and the vehicle-treated group. *P<0.05. Error bars denote SEM.
In the NOR task (Figure 5c), drug-naive mice explored the novel object more than the familiar objects, with a recognition index of 0.23±0.05. However, the recognition index of PCP-treated mice was significantly decreased and they displayed no preference for the novel object (recognition index −0.03±0.07), suggesting a profound deficit in recognition memory. Interestingly, a single injection of VU0453595 in PCP-treated mice rescued recognition memory to control levels, with a recognition index of 0.17±0.05 at the highest dose (Figure 5c). Thus, no significant difference was observed between 1, 3, and 10 mg/kg VU0453595-administered PCP-treated mice and the vehicle-treated mice (p>0.05). Control studies demonstrated that 10 mg/kg VU0453595 had no effect on recognition index in saline-treated mice (0.22±0.03) as compared with vehicle-treated controls. A one-way ANOVA revealed a significant effect of dose on recognition index (p=0.04; F=2.15; R2=0.1254). These data demonstrate the ability of the selective M1 PAM VU0453595 to restore impairments in both cognitive function and negative symptoms of a preclinical rodent model of schizophrenia.
DISCUSSION
The results described above show that mLTD induced both by the exogenous agonist CCh and endogenously released ACh in the rodent PFC is dependent on M1 activation and can be potentiated by the M1 PAM VU0453595. Moreover, this form of plasticity is compromised following transient blockade of NMDARs during early adolescent development by repeated PCP exposure in juvenile mice. Interestingly, these deficits in synaptic plasticity in the PFC can be rescued by incubation of acute slices with the highly selective M1 PAM VU0453595. In addition, behavioral deficits in PCP-treated mice, thought to represent examples of the negative and cognitive symptoms of schizophrenia, were also reversed following an acute treatment of a single dose of the M1 PAM. Taken together with previous clinical studies in schizophrenia patients and preclinical studies in rodent models, these data form a strong argument for the functional relevance of muscarinic plasticity in schizophrenia-related behavioral deficits and support the hypothesis that highly selective M1 PAMs may provide therapeutic efficacy in treatment of some aspects of the negative and cognitive symptoms in schizophrenia patients.
The present findings are especially interesting in light of multiple previous studies that cholinergic signaling is disrupted in the PFC and other cortical and limbic forebrain regions of schizophrenia patients (Berman et al, 2007; Dean et al, 2002; Raedler et al, 2007; Scarr et al, 2009a; Zavitsanou et al, 2004). Of particular interest is the recent finding that a defined subset of schizophrenia patients display decreases in M1 levels in PFC and other cortical and forebrain regions (Dean et al, 2002; Gibbons et al, 2013). Interestingly, studies using brain autopsies from a broad range of schizophrenia patients show reduced methylation of the promoter for the gene encoding the M1 mAChR subtype that may contribute to the reduced M1 expression in their brain (Scarr et al, 2013). However, the subset of schizophrenia patients who show the most profound loss of cortical M1 protein also show increases in an identified microRNA (miR-107) that contributes to the loss of M1 (Scarr et al, 2013). In the future, it will be important to develop a biomarker strategy, such as a selective M1 positron emission tomography (PET) ligand, that could be used to identify this subset of schizophrenia patients in whom deficits in M1 expression are most likely to contribute to the pathophysiology underlying their disease, in the hope that potentiating signaling of the remaining M1 receptors by M1 PAMs may be especially effective in reducing symptoms of these patients.
The PFC has long been considered a seat for several cognitive and emotional functions and is also implicated in various disorders that are characterized by cognitive and emotional dysfunction including schizophrenia. In addition, various studies have implicated M1 in regulating PFC function (Anagnostaras et al, 2003; Shirey et al, 2009). Our finding that M1-mediated plasticity in PFC is highly dysregulated in a rodent model of schizophrenia and that an M1 PAM can restore mLTD and also reverse behavioral deficits in this model provides substantial support for a number of previous preclinical and clinical studies demonstrating dysfunctional plasticity in the PFC. Moreover, the deficit in mLTD provides a potential explanation for the key pathological feature of increased activity of the PFC pyramidal neurons (Jodo, 2013; Jodo et al, 2005; Suzuki et al, 2002) in PCP-treated rodent models. This selective increase in activation of PFC neurons is known to be mediated primarily by inputs from the hippocampo-PFC pathway (Jodo, 2013; Jodo et al, 2005; Kamiyama et al, 2011; Thomases et al, 2014) where LTD is reduced and induction of LTP is enhanced (Kamiyama et al, 2011; Thomases et al, 2014). Previous studies reveal that mLTD in PFC is observed when specifically stimulating the hippocampo-prefrontal pathway (Lopes-Aguiar et al, 2013; Parent et al, 2010; Wang and Yuan, 2009). Thus, it is possible that loss of M1-mediated mLTD at the hippocampo-PFC synapse plays a major role in regulating PFC activation by hippocampal afferents under normal conditions, and that loss of this function contributes to the increased activation of the PFC and resultant behavioral outcomes of dysfunctional recognition memory and social interaction in this rodent model. As normal PFC function is essential for both social interaction (Elliott et al, 2012; Shah and Treit, 2003; Wall et al, 2012) and recognition memory (Bekinschtein and Weisstaub, 2014; Watson et al, 2012) in rodents, this study provides the first direct evidence for a potential role of impaired M1-mediated mLTD in PFC as an underlying cause of these behavioral deficits.
It is clear from our studies that the deficit in mLTD in PCP-treated mice cannot be explained by a general loss of M1 function in the pyramidal neurons of PFC as repeated PCP treatment does not affect CCh-induced increases in sEPSC or generation of inward currents, both of which are M1 dependent (Shirey et al, 2009). This strongly indicates that the mechanisms governing mLTD can be entirely different from the ones that govern M1-dependent changes in sEPSC frequency or M1-mediated inward currents. It is possible that downstream signaling pathways governing mLTD could be different from those governing the M1-mediated changes in synaptic transmission and inward current in pyramidal neurons. In addition, these different responses may be mediated by M1 expressed in different cell populations. One can also argue that this mLTD deficit is a general dysfunction in overall plasticity-related mechanisms in the PFC. However, we found that glutamatergic LTD mediated by the group II mGlu receptor mGlu3 (Walker et al, 2015) is intact in the PFC after PCP treatment, indicating PFC synapses can still undergo plastic changes in these mice. It is possible that mLTD and group II mGlu receptor-mediated LTD can reflect plasticity in distinct synapses in the PFC, thus serving distinct functions, and repeated PCP treatment leads to selective mLTD deficits and deficits in functions only related to this form of muscarinic plasticity.
In addition, it is important to consider these studies in the context of previous clinical studies with the orthosteric mAChR agonist, xanomeline, in schizophrenia patients (Shekhar et al, 2008). Although xanomeline failed to advance in clinical development because of peripheral adverse effects mediated by M2 and M3 activation, this compound had robust efficacy in reducing positive as well as negative symptoms and cognitive disturbances in schizophrenia patients. Extensive studies with novel, highly selective M4 PAMs suggest that selective potentiation of M4 has multiple effects in rodent models that predict efficacy in reducing positive symptoms (Byun et al, 2014; Chan et al, 2008). Thus, it is possible that activation of M4 could play a dominant role in xanomeline’s ability to reduce psychosis in schizophrenia patients, whereas M1 may be more important in mediating xanomeline’s efficacy in reducing negative and cognitive symptoms of schizophrenia.
At present, very few studies have focused on understanding the cellular mechanisms underlying mLTD in the PFC. M1 is a signaling partner for NMDARs and activation of M1 can potentiate NMDAR currents (Marino et al, 1998). However, previous studies in rodents have shown that mLTD in the PFC is NMDAR independent (Caruana et al, 2011; Wang and Yuan, 2009). The mLTD is also independent of nitric oxide but heavily dependent on activation of protein kinase C (Caruana et al, 2011). There is contrasting evidence in the role of postsynaptic Ca2+ in mLTD. Although one study has shown it to be heavily dependent on voltage-gated Ca2+ channels and postsynaptic Ca2+ levels (Wang and Yuan, 2009), others have reported that postsynaptic chelation of calcium by BAPTA does not influence mLTD in the PFC (Caruana et al, 2011). Of particular interest in the current studies was the observation of a consistent increase in PPR during expression of mLTD, with no changes in PPR in conditions that failed to produce mLTD. As changes in PPR are often linked to an involvement of presynaptic mechanisms (Schulz et al, 1994), it is possible that this form of M1-mediated LTD has a significant presynaptic component. This is in contrast to some previously reported examples of LTD that are mediated by postsynaptic changes in AMPA receptor trafficking (Casimiro et al, 2011; Snyder et al, 2001). A presynaptic component could be mediated by direct M1 activation in presynaptic terminals or via a retrograde messenger system activated by postsynaptic M1 receptors. However, it is also possible that M1 activation could induce changes in cortical network properties, leading to an overall depression in the PFC output. It is known that M1 activation can enhance excitability in the GABAergic interneurons of the PFC (Yi et al, 2014), and it is conceivable that M1 activation by high concentrations of CCh or a combination of the M1 PAM VU0453595 and CCh leads to a long-term enhancement of GABAergic interneuron-mediated inhibitory tone in the PFC and thereby leads to sustained depression of the excitatory output in PFC. Hence, the loss in mLTD in the PCP-treated mice could be related to a dysfunction in the muscarinic regulation of GABAergic neurotransmission in the PFC. This could also help explain our finding that M1 function in pyramidal neurons remains intact in the PFC of the PCP-treated mice. Indeed, there is evidence that shows that both acute and repeated NMDA receptor antagonism can lead to a marked loss in GABAergic neurotransmission in the brain including the frontal cortex (Brigman et al, 2009; Jentsch et al, 1998; Tanibuchi et al, 2009). For instance, loss of LTD at the hippocampo-PFC synapse has been postulated to be due to a failure in the recruitment of GABAergic interneurons in the PFC by the hippocampal inputs following repeated PCP treatment (Thomases et al, 2014). Finally, it is likely that rescue of mLTD with the M1 PAM in the PCP-treated mice could be due to its potentiation of the dysfunctional GABAergic network in the PFC. However, the exact cellular mechanism for this form of muscarinic plasticity is yet to be determined and needs further extensive studies to elucidate the mechanism.
In conclusion, this study suggests that M1 PAMs may provide an exciting new therapeutic approach for reducing the negative and cognitive symptoms of schizophrenia. These data build on strong clinical studies suggesting that schizophrenia patients display deficits in cortical LTD and decreased cortical M1 expression, and suggest that the established efficacy of less selective mAChR agonists in reducing cognitive and negative symptoms may be achieved using selective allosteric modulators of M1. In addition, the finding that M1 PAMs may reverse deficits in cortical LTD in a mouse model that parallel deficits in cortical LTD observed using noninvasive approaches in schizophrenia patients raises the possibility that this could provide a useful translational biomarker to evaluate effects of M1 PAMs in schizophrenia patients. Finally, it is important to note that Merck recently advanced an M1-selective PAM into clinical development for testing in patients suffering from Alzheimer’s disease (AD) (Merck Sharp and Dohme, 2014), and other companies are making similar efforts as an approach to improving cognition in AD patients. The present data, combined with previous clinical studies, provide a strong basis for evaluating these M1 PAMs for potential efficacy in improving cognitive function and reducing negative symptoms in schizophrenia patients.
FUNDING AND DISCLOSURE
Drs Wood, Melancon, Stauffer, Lindsley, Conn, and Niswender and Mr Poslusney are inventors on patents that protect different classes of M1 PAMs. Dr Daniels has received compensation as a member of the scientific advisory board of the Sigma-Aldrich Company and through consulting for Agios Pharmaceuticals Company and the Michael J Fox Foundation. Dr Jones received research support from Bristol Myers Squibb, Johnson & Johnson, and AstraZeneca. Dr Lindsley's work has been funded by the NIH, Bristol-Myers Squibb, AstraZeneca, Michael J Fox Foundation, as well as Seaside Therapeutics. He has consulted for AbbVie and received compensation. Dr Conn has been funded by NIH, Johnson & Johnson, AstraZeneca, Bristol-Myers Squibb, Michael J Fox Foundation, and Seaside Therapeutics. He has consulted over the past 3 years for Pfizer, Cambridge, and Millipore Corporation and received compensation. Over the past 3 years, he has served on the Scientific Advisory Boards of Seaside Therapeutics, Michael J Fox Foundation, Stanley Center for Psychiatric Research Broad Institute (MIT/Harvard), Karuna Pharmaceuticals, Lieber Institute for Brain Development Johns Hopkins University, Clinical Mechanism (POCM) and Proof of Concept (POC) Consortium, and Neurobiology Foundation for Schizophrenia and Bipolar Disorder. Drs Ghoshal, Rook, Dickerson, Jalan-Sakrikar, Noetzel, and Xiang, and Mr Roop, Morrison, Lamsal, and Poslusney declare no conflict of interest.
References
Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM et al (2003). Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nature Neurosci 6: 51–58.
Barak S, Weiner I (2011). The M(1)/M(4) preferring agonist xanomeline reverses amphetamine-, MK801- and scopolamine-induced abnormalities of latent inhibition: putative efficacy against positive, negative and cognitive symptoms in schizophrenia. Int J Neuropsychopharmacol 14: 1233–1246.
Bekinschtein P, Weisstaub N (2014). Role of PFC during retrieval of recognition memory in rodents. J Physiol Paris 108: 252–255.
Berman JA, Talmage DA, Role LW (2007). Cholinergic circuits and signaling in the pathophysiology of schizophrenia. Int Rev Neurobiol 78: 193–223.
Brigman JL, Ihne J, Saksida LM, Bussey TJ, Holmes A (2009). Effects of subchronic phencyclidine (PCP) treatment on social behaviors, and operant discrimination and reversal learning in C57BL/6J mice. Front Behav Neurosci 3: 2.
Byun NE, Grannan M, Bubser M, Barry RL, Thompson A, Rosanelli J et al (2014). Antipsychotic drug-like effects of the selective M4 muscarinic acetylcholine receptor positive allosteric modulator VU0152100. Neuropsychopharmacology 39: 1578–1593.
Caruana DA, Warburton EC, Bashir ZI (2011). Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J Neurosci 31: 18464–18478.
Casimiro TM, Sossa KG, Uzunova G, Beattie JB, Marsden KC, Carroll RC (2011). mGluR and NMDAR activation internalize distinct populations of AMPARs. Mol Cell Neurosci 48: 161–170.
Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC et al (2008). Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA 105: 10978–10983.
Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E (2002). Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry 7: 1083–1091.
Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, Byun NE et al (2012). Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci 32: 8532–8544.
Elliott R, Lythe K, Lee R, McKie S, Juhasz G, Thomas EJ et al (2012). Reduced medial prefrontal responses to social interaction images in remitted depression. Arch Gen Psychiatry 69: 37–45.
Ghoshal A, Conn PJ (2015). The hippocampo-prefrontal pathway: a possible therapeutic target for negative and cognitive symptoms of schizophrenia. Future Neurol 10: 115–128.
Gibbons AS, Scarr E, Boer S, Money T, Jeon WJ, Felder C et al (2013). Widespread decreases in cortical muscarinic receptors in a subset of people with schizophrenia. Int J Neuropsychopharmacol 16: 37–46.
Hasan A, Bergener T, Nitsche MA, Strube W, Bunse T, Falkai P et al (2013). Impairments of motor-cortex responses to unilateral and bilateral direct current stimulation in schizophrenia. Front Psychiatry 4: 121.
Hashimoto K, Ishima T, Fujita Y, Matsuo M, Kobashi T, Takahagi M et al (2008). Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the novel selective alpha7 nicotinic receptor agonist SSR180711. Biol Psychiatry 63: 92–97.
Jentsch JD, Taylor JR, Roth RH (1998). Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress- and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology 19: 105–113.
Jodo E (2013). The role of the hippocampo-prefrontal cortex system in phencyclidine-induced psychosis: a model for schizophrenia. J Physiol Paris 107: 434–440.
Jodo E, Suzuki Y, Katayama T, Hoshino KY, Takeuchi S, Niwa S et al (2005). Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampo-prefrontal pathway. Cereb Cortex 15: 663–669.
Kamiyama H, Matsumoto M, Otani S, Kimura SI, Shimamura KI, Ishikawa S et al (2011). Mechanisms underlying ketamine-induced synaptic depression in rat hippocampus-medial prefrontal cortex pathway. Neuroscience 177: 159–169.
Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y (2007). Activation of medial prefrontal cortex neurons by phencyclidine is mediated via AMPA/kainate glutamate receptors in anesthetized rats. Neuroscience 150: 442–448.
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991). Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11: 3218–3226.
Lewis DA, Lieberman JA (2000). Catching up on schizophrenia: natural history and neurobiology. Neuron 28: 325–334.
Lopes-Aguiar C, Bueno-Junior LS, Ruggiero RN, Romcy-Pereira RN, Leite JP (2013). NMDA receptor blockade impairs the muscarinic conversion of sub-threshold transient depression into long-lasting LTD in the hippocampus-prefrontal cortex pathway in vivo: correlation with gamma oscillations. Neuropharmacology 65: 143–155.
Manoach DS (2003). Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Res 60: 285–298.
Merck Sharp and Dohme (2014). Placebo-controlled crossover study to evaluate donepezil and mk-3134 for reversal of cognitive impairment associated with scopolamine administration (3134-005)(COMPLETED). Available from https://clinicaltrials.gov/ct2/show/NCT01181310?term=muscarinic+and+alzheimer+and+merck&rank=1.
Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998). Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci USA 95: 11465–11470.
Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL et al (2010). Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128: 419–432.
Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM (2014). Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol 24: 822–835.
Parent MA, Wang L, Su J, Netoff T, Yuan LL (2010). Identification of the hippocampal input to medial prefrontal cortex in vitro. Cereb Cortex 20: 393–403.
Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B (2007). Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry 12: 232–246.
Santini MA, Ratner C, Aznar S, Klein AB, Knudsen GM, Mikkelsen JD (2013). Enhanced prefrontal serotonin 2A receptor signaling in the subchronic phencyclidine mouse model of schizophrenia. J Neurosci Res 91: 634–641.
Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B (2009a). Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry 14: 1017–1023.
Scarr E, Craig JM, Cairns MJ, Seo MS, Galati JC, Beveridge NJ et al (2013). Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Transl Psychiatry 3: e230.
Scarr E, Dean B (2009b). Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother 9: 73–86.
Schulz PE, Cook EP, Johnston D (1994). Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci 14: 5325–5337.
Shah AA, Treit D (2003). Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res 969: 183–194.
Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE et al (2009). A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76: 356–368.
Shekhar A, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mallinckrodt C et al (2008). Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry 165: 1033–1039.
Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP et al (2009). A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29: 14271–14286.
Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF (2001). Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci 4: 1079–1085.
Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB et al (2001). The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther 299: 782–792.
Strube W, Bunse T, Nitsche MA, Wobrock T, Aborowa R, Misewitsch K et al (2014). Smoking restores impaired LTD-like plasticity in schizophrenia: a transcranial direct current stimulation study. Neuropsychopharmacology 40: 822–830.
Suzuki Y, Jodo E, Takeuchi S, Niwa S, Kayama Y (2002). Acute administration of phencyclidine induces tonic activation of medial prefrontal cortex neurons in freely moving rats. Neuroscience 114: 769–779.
Tanibuchi Y, Fujita Y, Kohno M, Ishima T, Takatsu Y, Iyo M et al (2009). Effects of quetiapine on phencyclidine-induced cognitive deficits in mice: a possible role of alpha1-adrenoceptors. Eur Neuropsychopharmacol 19: 861–867.
Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY (2014). Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci 34: 9059–9066.
Veselinovic T, Vernaleken I, Janouschek H, Kellermann T, Paulzen M, Cumming P et al (2014). Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology 232: 1607–1617.
Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM et al (2015). Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci USA 112: 1196–1201.
Wall VL, Fischer EK, Bland ST (2012). Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol Behav 107: 440–450.
Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T (2007). Synergistic effect of combined treatment with risperidone and galantamine on phencyclidine-induced impairment of latent visuospatial learning and memory: role of nAChR activation-dependent increase of dopamine D1 receptor-mediated neurotransmission. Neuropharmacology 53: 379–389.
Wang L, Yuan LL (2009). Activation of M2 muscarinic receptors leads to sustained suppression of hippocampal transmission in the medial prefrontal cortex. J Physiol 587: 5139–5147.
Watson DJ, Loiseau F, Ingallinesi M, Millan MJ, Marsden CA, Fone KC (2012). Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37: 770–786.
Woodward ND, Duffy B, Karbasforoushan H (2013). Prefrontal cortex activity during response selection predicts processing speed impairment in schizophrenia. J Int Neuropsychol Soc 19: 782–791.
Yi F, Ball J, Stoll KE, Satpute VC, Mitchell SM, Pauli JL et al (2014). Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol 592: 3463–3494.
Zavitsanou K, Katsifis A, Mattner F, Huang XF (2004). Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology 29: 619–625.
Acknowledgements
This work was supported by funding from the National Institute of Mental Health (grants U01 MH087965, R01 MH073676, 2RO1 MH082867, and U54 MH0845659). We sincerely thank James T Maksymetz for his diligent help in conducting some of the electrophysiological studies.
Author contributions
AG and JMR: designed, performed experiments, analyzed data, and wrote the article; JWD and GNR: acquired data for behavioral experiments; NJ-S, AL, and MJN: acquired data for molecular screening experiments; MSP, MRW, BJM, and SRS: performed chemical synthesis of compounds and wrote the article; RDM: acquired data for pharmacokinetic studies; JSD: supervised pharmacokinetic studies; CMN: supervised molecular studies and wrote the article; ZX: supervised electrophysiological studies and wrote the article; CKJ: supervised behavioral studies; CWL: supervised the chemistry studies and wrote the article; and PJC: supervised and designed experiments and wrote the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Ghoshal, A., Rook, J., Dickerson, J. et al. Potentiation of M1 Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model. Neuropsychopharmacol 41, 598–610 (2016). https://doi.org/10.1038/npp.2015.189
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.189
This article is cited by
-
mGlu1-mediated restoration of prefrontal cortex inhibitory signaling reverses social and cognitive deficits in an NMDA hypofunction model in mice
Neuropsychopharmacology (2022)
-
Clinical and Preclinical Evidence for M1 Muscarinic Acetylcholine Receptor Potentiation as a Therapeutic Approach for Rett Syndrome
Neurotherapeutics (2022)
-
Effects of muscarinic M1 receptor stimulation on reinforcing and neurochemical effects of cocaine in rats
Neuropsychopharmacology (2020)
-
Modulation of arousal and sleep/wake architecture by M1 PAM VU0453595 across young and aged rodents and nonhuman primates
Neuropsychopharmacology (2020)
-
Biased M1-muscarinic-receptor-mutant mice inform the design of next-generation drugs
Nature Chemical Biology (2020)