Abstract
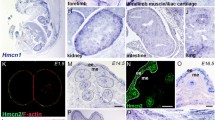
Collagen IV comprises the predominant protein network of basement membranes, a specialized extracellular matrix, which underlie epithelia and endothelia. These networks assemble through oligomerization and covalent crosslinking to endow mechanical strength and shape cell behavior through interactions with cell-surface receptors. A recently discovered sulfilimine (S=N) bond between a methionine sulfur and hydroxylysine nitrogen reinforces the collagen IV network. We demonstrate that peroxidasin, an enzyme found in basement membranes, catalyzes formation of the sulfilimine bond. Drosophila peroxidasin mutants have disorganized collagen IV networks and torn visceral muscle basement membranes, pointing to a critical role for the enzyme in tissue biogenesis. Peroxidasin generates hypohalous acids as reaction intermediates, suggesting a paradoxically anabolic role for these usually destructive oxidants. This work highlights sulfilimine bond formation as what is to our knowledge the first known physiologic function for peroxidasin, a role for hypohalous oxidants in tissue biogenesis, and a possible role for peroxidasin in inflammatory diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hynes, R.O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Yurchenco, P.D. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3, a004911 (2011).
Khoshnoodi, J., Pedchenko, V. & Hudson, B.G. Mammalian collagen IV. Microsc. Res. Tech. 71, 357–370 (2008).
Vanacore, R. et al. A sulfilimine bond identified in collagen IV. Science 325, 1230–1234 (2009).
Pedchenko, V. et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N. Engl. J. Med. 363, 343–354 (2010).
Fessler, L.I. & Fessler, J.H. Identification of the carboxyl peptides of mouse procollagen IV and its implications for the assembly and structure of basement membrane procollagen. J. Biol. Chem. 257, 9804–9810 (1982).
Nelson, R.E. et al. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 13, 3438–3447 (1994).
Adeniyi-Jones, S.K. & Karnovsky, M.L. Oxidative decarboxylation of free and peptide-linked amino acids in phagocytizing guinea pig granulocytes. J. Clin. Invest. 68, 365–373 (1981).
Nagasaka, A. & Hidaka, H. Effect of antithyroid agents 6-propyl-2-thiouracil and 1-mehtyl-2-mercaptoimidazole on human thyroid iodine peroxidase. J. Clin. Endocrinol. Metab. 43, 152–158 (1976).
Alexander, N.M. Iodide peroxidase in rat thyroid and salivary glands and its inhibition by antithyroid compounds. J. Biol. Chem. 234, 1530–1533 (1959).
Weiss, S.J., Klein, R., Slivka, A. & Wei, M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J. Clin. Invest. 70, 598–607 (1982).
Tang, S.S., Trackman, P.C. & Kagan, H.M. Reaction of aortic lysyl oxidase with β-aminopropionitrile. J. Biol. Chem. 258, 4331–4338 (1983).
Candi, E. et al. Biochemical, structural, and transglutaminase substrate properties of human loricrin, the major epidermal cornified cell envelope protein. J. Biol. Chem. 270, 26382–26390 (1995).
Ortiz de Montellano, P.R., David, S.K., Ator, M.A. & Tew, D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry 27, 5470–5476 (1988).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Edn Engl. 41, 2596–2599 (2002).
Obinger, C. Chemistry and biology of human peroxidases. Arch. Biochem. Biophys. 445, 197–198 (2006).
Dypbukt, J.M. et al. A sensitive and selective assay for chloramine production by myeloperoxidase. Free Radic. Biol. Med. 39, 1468–1477 (2005).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050–15055 (2001).
Gotenstein, J.R. et al. The C. elegans peroxidasin PXN-2 is essential for embryonic morphogenesis and inhibits adult axon regeneration. Development 137, 3603–3613 (2010).
Gupta, M.C., Graham, P.L. & Kramer, J.M. Characterization of α1(IV) collagen mutations in Caenorhabditis elegans and the effects of α1 and α2(IV) mutations on type IV collagen distribution. J. Cell Biol. 137, 1185–1196 (1997).
Cheng, G., Salerno, J.C., Cao, Z., Pagano, P.J. & Lambeth, J.D. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic. Biol. Med. 45, 1682–1694 (2008).
Coupry, I. et al. Ophthalmological features associated with COL4A1 mutations. Arch. Ophthalmol. 128, 483–489 (2010).
Favor, J. et al. Type IV procollagen missense mutations associated with defects of the eye, vascular stability, the brain, kidney function and embryonic or postnatal viability in the mouse, Mus musculus: an extension of the Col4a1 allelic series and the identification of the first two Col4a2 mutant alleles. Genetics 175, 725–736 (2007).
Gould, D.B., Marchant, J.K., Savinova, O.V., Smith, R.S. & John, S.W. Col4a1 mutation causes endoplasmic reticulum stress and genetically modifiable ocular dysgenesis. Hum. Mol. Genet. 16, 798–807 (2007).
Labelle-Dumais, C. et al. COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 7, e1002062 (2011).
Van Agtmael, T. et al. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum. Mol. Genet. 14, 3161–3168 (2005).
Péterfi, Z. et al. Peroxidasin is secreted and incorporated into the extracellular matrix of myofibroblasts and fibrotic kidney. Am. J. Pathol. 175, 725–735 (2009).
Beal, J.L., Foster, S.B. & Ashby, M.T. Hypochlorous acid reacts with the N-terminal methionines of proteins to give dehydromethionine, a potential biomarker for neutrophil-induced oxidative stress. Biochemistry 48, 11142–11148 (2009).
Peskin, A.V., Turner, R., Maghzal, G.J., Winterbourn, C.C. & Kettle, A.J. Oxidation of methionine to dehydromethionine by reactive halogen species generated by neutrophils. Biochemistry 48, 10175–10182 (2009).
Armesto, X.L., Canle, M., Fernandez, M.I., Garcia, M.V. & Santaballa, J.A. First steps in the oxidation of sulfur-containing amino acids by hypohalogenation: very fast generation of intermediate sulfenyl halides and halosulfonium cations. Tetrahedron 56, 1103–1109 (2000).
Lavine, T.F. The formation, resolution, and optical properties of the diastereoisomeric sulfoxides derived from L-methionine. J. Biol. Chem. 169, 477–491 (1947).
Huwiler, M., Burgi, U. & Kohler, H. Mechanism of enzymatic and non-enzymatic tyrosine iodination. Inhibition by excess hydrogen peroxide and/or iodide. Eur. J. Biochem. 147, 469–476 (1985).
Blair-Johnson, M., Fiedler, T. & Fenna, R. Human myeloperoxidase: structure of a cyanide complex and its interaction with bromide and thiocyanate substrates at 1.9 A resolution. Biochemistry 40, 13990–13997 (2001).
Andrews, P.C. & Krinsky, N.I. A kinetic analysis of the interaction of human myeloperoxidase with hydrogen peroxide, chloride ions, and protons. J. Biol. Chem. 257, 13240–13245 (1982).
Taurog, A. & Dorris, M.L. Myeloperoxidase-catalyzed iodination and coupling. Arch. Biochem. Biophys. 296, 239–246 (1992).
Garver, L.S., Xi, Z. & Dimopoulos, G. Immunoglobulin superfamily members play an important role in the mosquito immune system. Dev. Comp. Immunol. 32, 519–531 (2008).
Zamocky, M., Jakopitsch, C., Furtmuller, P.G., Dunand, C. & Obinger, C. The peroxidase-cyclooxygenase superfamily: reconstructed evolution of critical enzymes of the innate immune system. Proteins 72, 589–605 (2008).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Touyz, R.M. & Briones, A.M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 34, 5–14 (2011).
Yokoyama, M. Oxidant stress and atherosclerosis. Curr. Opin. Pharmacol. 4, 110–115 (2004).
Bai, Y.P. et al. Role of VPO1, a newly identified heme-containing peroxidase, in ox-LDL induced endothelial cell apoptosis. Free Radic. Biol. Med. 51, 1492–1500 (2011).
Shi, R. et al. Involvement of vascular peroxidase 1 in angiotensin II–induced vascular smooth muscle cell proliferation. Cardiovasc. Res. 91, 27–36 (2011).
Brandes, R.P. Vascular peroxidase 1/peroxidasin: a complex protein with a simple function? Cardiovasc. Res. 91, 1–2 (2011).
Lau, D. & Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 111, 16–26 (2006).
Li, H. et al. Microbicidal activity of vascular peroxidase 1 in human plasma via generation of hypochlorous acid. Infect. Immunity 80, 2528–2537 (2012).
Kramer, R.H., Bensch, K.G., Davison, P.M. & Karasek, M.A. Basal lamina formation by cultured microvascular endothelial cells. J. Cell Biol. 99, 692–698 (1984).
Weber, S., Dölz, R., Timpl, R., Fessler, J.H. & Engel, J. Reductive cleavage and reformation of the interchain and intrachain disulfide bonds in the globular hexameric domain NC1 involved in network assembly of basement membrane collagen (type IV). Eur. J. Biochem. 175, 229–236 (1988).
Acknowledgements
This work was supported by the US National Institutes of Health (RO1 DK18381, DK18381-38S1 and 2PO1 DK065123 to B.G.H.), the Mount Desert Island Biological Laboratory Salisbury Cove Research Fund and F.H. Epstein Fellowship (to B.G.H.), the Vanderbilt Division of Nephrology Faculty Development Fund (to R.M.V.) and a Vanderbilt Physician Scientist Development Award (to G.B.). We are grateful to K.L. Rose and W.H. McDonald of the Mass Spectrometry Resource Center at Vanderbilt University for assistance with mass spectrometry, which was supported in part by the Vanderbilt Academic Venture Capital Fund. We acknowledge A. Chisholm and A. Page-McCaw for fruitful discussions during the writing of this manuscript. We thank M. Geizst (Semmelweis University) for the human peroxidasin coding sequence and L. Cooley (Yale University) for the vikingG454 protein trap Drosophila line. P. Todd, N. Danylevych and C. Venkov provided technical assistance.
Author information
Authors and Affiliations
Contributions
G.B. conducted, designed and analyzed data from the PFHR-9 cell culture experiments, purified collagen IV NC1 hexamers from Drosophila and conducted western blotting experiments on Drosophila mutants. C.F.C. conducted mechanistic experiments involving hypohalous acids and peroxidasin. R.M.V. conducted MS and analysis. L.I.F. prepared Drosophila materials, and C.K.-C. performed Drosophila genetics and confocal microscopy. I.A.E.-T. performed overlay experiments involving peroxidasin and other peroxidases. M.R. isolated collagen IV NC1 hexamers and sulfilimine-crosslinked peptides for further analysis. J.-S.K. isolated human peroxidasin expressing HEK293 stable cell lines, and V.P. established the PFHR-9 cell culture system for these studies. L.I.F. generated Drosophila mutant larvae, antibodies and protein reagents. L.I.F., J.H.F. and B.G.H. designed the study and wrote the paper along with G.B. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Results (PDF 1358 kb)
Rights and permissions
About this article
Cite this article
Bhave, G., Cummings, C., Vanacore, R. et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol 8, 784–790 (2012). https://doi.org/10.1038/nchembio.1038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1038
This article is cited by
-
Anastellin impacts on the processing of extracellular matrix fibronectin and stimulates release of cytokines from coronary artery smooth muscle cells
Scientific Reports (2022)
-
Complexities of the glomerular basement membrane
Nature Reviews Nephrology (2021)
-
Hydrogen selenide, a vital metabolite of sodium selenite, uncouples the sulfilimine bond and promotes the reversal of liver fibrosis
Science China Life Sciences (2021)
-
Peroxidasin Enhances Basal Phenotype and Inhibits Branching Morphogenesis in Breast Epithelial Progenitor Cell Line D492
Journal of Mammary Gland Biology and Neoplasia (2021)
-
Novel PXDN biallelic variants in patients with microphthalmia and anterior segment dysgenesis
Journal of Human Genetics (2020)